��Ŀ����
����Ŀ����1����ͬ������,ij�������ռ���1���CO2��3���H2,��������CO2��H2�����ʵ���֮����__________;���������ռ���CO2��H2���������������������,��CO2��H2������֮����_____________��
��2��0.01 molij���ʵ�����Ϊ1.08g�������ʵ�Ħ������Ϊ_________��
��3����֪A�Ƕ��۽���,82 g�ý������������к���6.02 x1023�����������,��������ε�Ħ������Ϊ________________��
���𰸡� 1:3 22:1 108 g��mol-1 164 g��mol-1
��������(1)ͬ��ͬѹ�£��������ʵ���֮�ȵ����������֮�ȣ���������CO2��H2�����ʵ���֮����1�����3���=1��3����CO2��H2��������ķ�����Ŀ��ȣ���������ʵ�����ȣ�����m=nM��֪����������֮��Ϊ44g/mol��2g/mol=22��1���ʴ�Ϊ��1��3��22��1��
(2)0.01molij���ʵ�����Ϊ1.08g�������ʵ�Ħ������Ϊ![]() =108g/mol���ʴ�Ϊ��108g/mol��
=108g/mol���ʴ�Ϊ��108g/mol��
(3)A�Ƕ��۽�������������ΪA(NO3)2��6.02��1023����������ӵ����ʵ���Ϊ�� ![]() =1mol����n[A(NO3)2]=
=1mol����n[A(NO3)2]= ![]() n(NO3-)=0.5mol���������ε�Ħ������Ϊ��
n(NO3-)=0.5mol���������ε�Ħ������Ϊ�� ![]() =164gmol-1���ʴ�Ϊ��164gmol-1��
=164gmol-1���ʴ�Ϊ��164gmol-1��

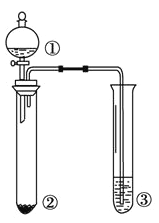
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ��ˮ | NaBr | ����KI��Һ | �����ԣ�Cl2>Br2>I2 | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | Br2�ı���Һ | ��м | AgNO3��Һ | �����嵥���������������� ����ȡ����Ӧ | |
D | ���� | Na2SO3 | KMnO4��Һ | SO2��ʹKMnO4��Һ��ɫ |
A. A B. B C. C D. D