��Ŀ����
���ú�̼������ϳ�ȼ���ǽ����ԴΣ������Ҫ��������֪CO(g)��2H2(g)  CH3OH(g)��Ӧ�����е������仯�����ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ���ǣ� ��
CH3OH(g)��Ӧ�����е������仯�����ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ���ǣ� ��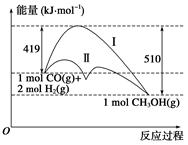
| A���÷�Ӧ�Ħ�H����91 kJ��mol��1 |
| B������������÷�Ӧ�Ħ�H��С |
| C����Ӧ�������������������������� |
| D������÷�Ӧ����Һ̬CH3OH����H���� |
C
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����б�������ȷ����
| A���κ���ʹ��ֵ����Ĺ��̶����Է����� |
| B����H��0����S��0�Ļ�ѧ��Ӧһ�����Է����� |
C����֪�Ȼ�ѧ����ʽ2SO2(g)��O2(g) 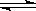 2SO3(g)��H����QkJ��mol��1��Q��0������2mol SO2(g) ��1mol O2(g) ����һ�ܱ������г�ַ�Ӧ��ų�Q kJ������ 2SO3(g)��H����QkJ��mol��1��Q��0������2mol SO2(g) ��1mol O2(g) ����һ�ܱ������г�ַ�Ӧ��ų�Q kJ������ |
| D��ϡ��Һ��1mol NaOH�ֱ��1mol CH3COOH��1molHNO3��Ӧ�����߷ų�������һ���� |
25�桢101kPa�£�̼�������ͼ����ȼ����������393.5 kJ��mol��1��285.8 kJ��mol��1
��890.3kJ��mol��1������ѡ������ȷ����
| A��2H2(g)��O2(g)��2H2O(l)��H����285.8kJ��mol��1 |
| B��CH4(g)��2O2(g)��CO2(g)��2H2O(l)��H��+890.3kJ��mol��1 |
| C��H2(g)��1/2O2(g)��H2O(g)��H����285.8kJ��mol��1 |
| D��C(s)��2H2(g)��CH4(g)��H����74.8 kJ��mol��1 |
����ͼʾ���Ӧ�������������
| A��ͼ1��ʾ��ij����������������Һ�м���NaOH��Һ���������������NaOH��Һ����Ĺ�ϵ |
| B��ͼ2��ʾ25��ʱ����0.1mol?L-1����ζ�20mL0.1mol?L-1NaOH��Һ��pH�������������ı仯 |
| C��ͼ3��ʾt��ʱϡ�ͱ������������Һ�����Եı仯 |
| D������ͼ4���ж�ij���淴Ӧ������Ӧ�����ȷ�Ӧ |
��˹������Ϊ�����ܻ�ѧ������һ�����Ϊ������ɣ�������̵���ЧӦ����ͬ�ġ�
��֪��H2O��g���� H2O��l�� ��H1 ����Q1 kJ��mol��1��Q1��0��
C2H5OH��g���� C2H5OH��l�� ��H2 ����Q2 kJ��mol��1��Q2��0��
C2H5OH��g����3O2��g����2CO2��g����3H2O��g�� ��H3 �� ��Q3 kJ��mol��1��Q3��0����ʹ23gҺ̬�Ҵ���ȫȼ�գ����ָ������£���ų�������Ϊ��kJ��
| A��Q1�� Q2��Q3 | B��0.5��Q1��Q2��Q3 �� |
| C��0.5 Q1��1.5 Q2��0.5Q3 | D��1.5 Q1��0.5 Q2��0.5Q3 |
�£�N2 H4����һ�ֿ����ڻ����ԭ��ص�ȼ�ϡ���֪��
N2��g��+2O2��g��=2NO2��g�� ��H=+67��7 kJ��mol ��
N2 H4��g��+O2��g��=N2��g��+2H3O��g�� ��H=��534 kJ��mol ��
����˵����ȷ����
| A����Ӧ���з�Ӧ�������е����������������������е������� |
| B��2N2 H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=��1000��3 kJ/mol |
| C�������缫��KOH��Һ���������Һ���ɷ�Ӧ����Ƶ�ȼ�ϵ�أ��为����ӦʽΪN2H4��4e��+4OH��=N2+4H2O |
| D�������缫��KOH��Һ���������Һ���ɷ�Ӧ����Ƶ�ȼ�ϵ�أ�����һ��ʱ���KOH��Һ��pH������ |
��֪�����Ȼ�ѧ����ʽ��
Na��(g)��Cl��(g)=NaCl(s)�� ��H
Na(s)�� Cl2(g)=NaCl(s)�� ��H1
Cl2(g)=NaCl(s)�� ��H1
Na(s)=Na(g)����H2��Na(g)��e��=Na��(g)����H3 Cl2(g)=Cl(g)����H4��Cl(g)��e��=Cl��(g)�� ��H5
Cl2(g)=Cl(g)����H4��Cl(g)��e��=Cl��(g)�� ��H5
��H�릤H1����H2����H3����H4����H5�Ĺ�ϵ��ȷ����(����)
| A����H����H1����H2����H3����H4����H5 |
| B����H����H1����H2����H3����H4����H5 |
| C����H����H1����H2����H3����H4����H5 |
| D����H����H2����H3����H4����H5����H1 |
��298 K��1.01��105 Pa��,��32 g SO2ͨ��750 mL 1 mol/L KOH��Һ�г�ַ�Ӧ����÷�Ӧ�ų�x kJ����������֪�ڸ�������,1 mol SO2ͨ��1 L 2 mol/L KOH��Һ�г�ַ�Ӧ�ų�y kJ����������SO2��KOH��Һ��Ӧ����KHSO3���Ȼ�ѧ����ʽ��ȷ���ǣ� ��
| A��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(4x-y)" kJ/mol |
| B��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(2x-y)" kJ/mol |
| C��SO2(g)+KOH(aq)=KHSO3(aq)��H="-(2y-x)" kJ/mol |
| D��2SO2(g)+2KOH(l)=2KHSO3(l)��H="-(8x-2y)" kJ/mol |
25 �桢101 kPa�£�̼������������������ǵ�ȼ����������393.5 kJ��mol��1��285.8 kJ��mol��1��890.3 kJ��mol��1��2 800 kJ��mol��1���������Ȼ�ѧ����ʽ��ȷ���ǣ���������
A��C��s����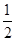 O2��g��=CO��g����H����393.5 kJ��mol��1 O2��g��=CO��g����H����393.5 kJ��mol��1 |
| B��2H2��g����O2��g��=2H2O��g����H����571.6 kJ��mol��1 |
| C��CH4��g����2O2��g��=CO2��g����2H2O��g����H����890.3 kJ��mol��1 |
D��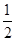 C6H12O6��s����3O2��g��=3CO2��g����3H2O��l����H����1 400 kJ��mol��1 C6H12O6��s����3O2��g��=3CO2��g����3H2O��l����H����1 400 kJ��mol��1 |