��Ŀ����
��֪�����Ȼ�ѧ����ʽ��
Na��(g)��Cl��(g)=NaCl(s)�� ��H
Na(s)�� Cl2(g)=NaCl(s)�� ��H1
Cl2(g)=NaCl(s)�� ��H1
Na(s)=Na(g)����H2��Na(g)��e��=Na��(g)����H3 Cl2(g)=Cl(g)����H4��Cl(g)��e��=Cl��(g)�� ��H5
Cl2(g)=Cl(g)����H4��Cl(g)��e��=Cl��(g)�� ��H5
��H�릤H1����H2����H3����H4����H5�Ĺ�ϵ��ȷ����(����)
| A����H����H1����H2����H3����H4����H5 |
| B����H����H1����H2����H3����H4����H5 |
| C����H����H1����H2����H3����H4����H5 |
| D����H����H2����H3����H4����H5����H1 |
B
����

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д���ӦA(g)��B(g)  C(g)��D(g)�����е������仯��ͼ��ʾ���ɴ˿��ж�
C(g)��D(g)�����е������仯��ͼ��ʾ���ɴ˿��ж�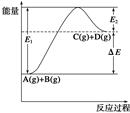
| A��1molA��1molB��ַ�Ӧ�������仯Ϊ��E |
| B�����������Ӧ�ӿ죬��E��С |
| C����Ӧ����ܼ���С����������ܼ��� |
| D����Ӧ�ﵽƽ��ʱ�������¶ȣ�A��ת�������� |
���ܼ��������ǵ�����ϢϢ��أ�������ܼ�����ÿһλ����Ӧ�����������оٴ벻������һҪ����ǣ� ��
| A��Ϊ�ƹ����ܵ�ʹ�ã���ҵ�Ͽɲ��õ��ˮ����ȡ�������� |
| B����ʯ���ѻ����ѽ⣬�ۺ�����ʯ����Դ |
| C���н��Ƶؿ���ú��ʯ�͡���Ȼ���ȿ�����Դ |
| D����ijЩ�Ͼ������ۻ����ٳ��� |
�к��ȵ���ֵ��57.3kJ/mol�������ᡢ����Һ��ϲ�������������57.3kJ���ǣ� ��
| A��1mol/L��ϡHCl��Һ��1mol/L��ϡNaOH��Һ |
| B��1mol/L��ϡH2SO4��Һ��1mol/L��ϡBa��OH��2��Һ |
| C��1 L 1mol/L��ϡHCl��Һ��2 L 1mol/L��ϡNaOH��Һ |
| D��1 L 1mol/L��ϡH2SO4��Һ��1 L 1mol/L��ϡBa��OH��2��Һ |
����˵�����ʾ������ȷ����(����)
| A����Ӧ��������������������������ʱ��һ�������Է����з�Ӧ |
B����֪��CH4(g)�� O2(g)=2H2O(l)��CO(g)����H����H��ʾCH4��ȼ���� O2(g)=2H2O(l)��CO(g)����H����H��ʾCH4��ȼ���� |
| C�����º�ѹʱ����2 mol A��1 mol BͶ���ܱ������У�������Ӧ��2A(g)��B(g)??2C(g)����ַ�Ӧ���÷ų�������ΪQ kJ����÷�Ӧ�Ħ�H����Q kJ/mol |
| D����4P(s������)=P4(s������)����H����139.2 kJ/mol����֪���ױȰ����ȶ� |
���ú�̼������ϳ�ȼ���ǽ����ԴΣ������Ҫ��������֪CO(g)��2H2(g)  CH3OH(g)��Ӧ�����е������仯�����ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ���ǣ� ��
CH3OH(g)��Ӧ�����е������仯�����ͼ��ʾ�����ߢ�����ߢ�ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ���ǣ� ��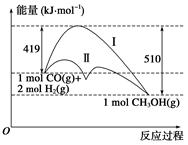
| A���÷�Ӧ�Ħ�H����91 kJ��mol��1 |
| B������������÷�Ӧ�Ħ�H��С |
| C����Ӧ�������������������������� |
| D������÷�Ӧ����Һ̬CH3OH����H���� |
�������ʼ�ķ�Ӧ,�������仯������ͼ����(����)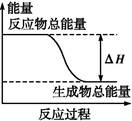
| A����������ڼ��������µķ�Ӧ |
| B�����ȵ�̼�������̼��Ӧ |
| C��Ba(OH)2��8H2O�����NH4Cl������ |
| D��̼��Ƶķֽ� |
��֪��2C(s)+O2(g) 2CO(g)����H="-221.0" kJ��mol-1
2CO(g)����H="-221.0" kJ��mol-1
��2H2(g)+O2(g) 2H2O(g)����H="-483.6" kJ��mol-1
2H2O(g)����H="-483.6" kJ��mol-1
��ӦC(s)+H2O(g) CO(g)+H2(g)�Ħ�HΪ(����)
CO(g)+H2(g)�Ħ�HΪ(����)
| A��+131.3 kJ��mol-1 | B��-131.3 kJ��mol-1 | C��-352.3 kJ��mol-1 | D��+262.6 kJ��mol-1 |
���ڷ�Ӧ��C2H4(g)=C2H2(g)��H2(g)��2CH4(g)=C2H4(g)��2H2(g)���������¶�ʱ�������ƶ�����C(s)��2H2(g)=CH4(g)����H1����2C(s)��H2(g)=C2H2(g)��H2����2C(s)��2H2(g)=C2H4(g)����H3����٢ڢ��Ц�H1����H2����H3�Ĵ�С˳��������ȷ����(����)
| A����H1>��H2>��H3 | B����H2>��H3>��H1 | C����H2>��H1>��H3 | D����H3>��H2>��H1 |