��Ŀ����
�������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2
�ܷ������·�Ӧ��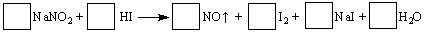
��1����ƽ������Ӧ����ʽ����ϵ�����뷽���С�
��2��������Ӧ���������� ������Ӧ����5 mol����ת�ƣ�������NO�ڱ�״���µ������ L��
��3������������Ӧ��������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У���ˮ���ڵ⻯�ص�����ֽ���۵��ۣ��ܰƣ���ʳ�ף�����ʵ�飬���м�����ʵ��� ��
| A���ۢ� | B���٢ڢ� | C���٢ڢ� | D���٢ڢۢ� |
��1��2NaNO2 + 4HI ��2NO + I2 +2 NaI +2 H2O ��2��NaNO2��112 ��3��C
���������������1�����ݵ�ʧ�����غ㷨��ƽ����ʽ������������NΪ+3�ۣ���Ӧ��Ϊ+2�ۣ�HI��IΪ-1�ۣ���Ӧ���Ϊ��������Ϊ0�ۣ����Դ�Ϊ2NaNO2 + 4HI ��2NO + I2 +2 NaI +2 H2O��
��2����������Ԫ�ػ��ϼ۽��͵����ʣ�������������NaNO2��ÿ����1molNO,��ת��1mol���ӣ�����Ӧ����5 mol����ת�ƣ�������5molNO,��״���µ������112L��
��3�����ݣ�2����֪���������������������£���������������Ϊ�ⵥ�ʣ��������۱���ɫ������ѡ���һ���Լ���C��
���㣺����������ԭ��Ӧ����ʽ����ƽ�����㣬���������жϣ����ʵļ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ѧ���ճ������о��й㷺��Ӧ�ã�����Ӧ���в��漰������ԭ��Ӧԭ������
| A��������[KAl(SO4)2��12H2O]��ˮ | B����ˮ����ȡ�� |
| C��������CrO3����˾���Ƿ�ƺ��ʻ | D��ҽ������˫��ˮ���� |
���û��ϼۺ���������Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�
��1���ӻ��ϼ۵ĽǶȿ���Ԥ�����ʵ����ʡ�
�ٽ�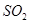 ͨ������
ͨ������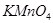 ��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
| A��S2- | B��S | C��SO32- | D��SO42- |
��ѡȡ���ʵ��Լ�֤��Na2SO3���л�ԭ�ԣ� ��д���÷�Ӧ�����ӷ���ʽΪ�� ��
��2�������ʷ���ĽǶȿ����Ʋ����ʵ����ʡ�
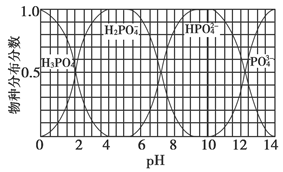
����֪����ʯ��MgO��Al2O3��SiO2��Fe2O3��ɡ��������ڼ������������ ��
����ȡһ������ʯ��������ʵ�飺
I���Ƚ������ڹ����������С����ˣ���������Ҫ�ɷ��� ��
II��������Һ�м���NaOH��Һ�����������ˣ������е���Ҫ�ɷ��� ��
 ��1��Q���ʵĵ���ʽΪ_______��
��1��Q���ʵĵ���ʽΪ_______��

 H2O2+BaCl2
H2O2+BaCl2 2H2O+O2��
2H2O+O2��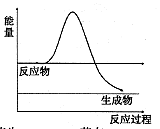
 ���뼾���Ĵ���
���뼾���Ĵ���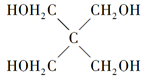 �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��