��Ŀ����
ˮ������֮Դ���������ǵ�����������أ��ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ�����ش��������⣺��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ
��2��H2O��������ԭ�Ӳ�ȡ����
��3��ˮ�������õ�һ��H+�γ�ˮ�������ӣ�H3O+�������������̵�������������������
A����ԭ�ӵ��ӻ����ͷ����˸ı� B��������״�����˸ı�
C��ˮ�����Ա������Ļ�ѧ���� D�����еļ��Ƿ����˸ı�
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ��δ��˳����������ľ���
������ͬ����
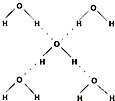
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ��������ͼ��ʾ������֪������������51kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11kJ/mol�����������������ġ����ܡ���
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ���д�����ɴ�������ӵ����ӷ���ʽ��
��7����֪����Ԫ�صĵ縺�����ݣ�H��2.1��O��3.5��F��4.0��OF2��ˮ������ṹ���ƣ���ˮ���ӵļ��Ա�OF2ǿ�ö࣬��ԭ���У���OF2����ԭ���������Թ¶Ե��ӣ�������FһO���й��õ��Ӷ�ƫ��F�������ļ��ԣ��ڴӵ縺���Ͽ���
��8�������±����ݣ���д�������߸����ԵĽ��ۣ�
| ���� | ���� ��kJ/mol�� |
���� ��pm�� |
���� | ���� | ���� | �۵㣨�棩 | �е㣨�棩 |
| H-C | 413 | 109 | 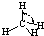 |
109.5�� | ���� | -183.7 | -128.0 |
| H-N | 391 | 101 | 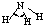 |
107�� | �� | -77.7 | -33.3 |
| H-O | 467 | 96 | 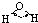 |
104.5�� | ˮ | 0.0 | 100.0 |
��������1������ԭ��������8��
��2�����ݼ۵��ӶԻ��������ж��ӻ���ʽ������ԭ����Ŀ�ͼ۵�����Ŀ�������ȵ����壻
��3��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3���ݴ˽��
��4�������ڷ��Ӿ��壬���þ���ͼ���жϾ���Ĺ��������Դ���������
��5��������=���»���+��������þ�̯������ˮ����������Ĺ�ϵ����������ȡ����»�������������ܣ�
��6������ͭ����ˮ��ͭ������ˮ�����˳���ɫ���������[Cu��H2O��4]2+�����������Ϊƽ�������Σ�
��7������Ԫ��֮��縺�Բ�ֵ������
��8������Խ�̣�����Խ���۷е�Խ�ߣ����Ӽ�������Խ��
��2�����ݼ۵��ӶԻ��������ж��ӻ���ʽ������ԭ����Ŀ�ͼ۵�����Ŀ�������ȵ����壻
��3��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3���ݴ˽��
��4�������ڷ��Ӿ��壬���þ���ͼ���жϾ���Ĺ��������Դ���������
��5��������=���»���+��������þ�̯������ˮ����������Ĺ�ϵ����������ȡ����»�������������ܣ�
��6������ͭ����ˮ��ͭ������ˮ�����˳���ɫ���������[Cu��H2O��4]2+�����������Ϊƽ�������Σ�
��7������Ԫ��֮��縺�Բ�ֵ������
��8������Խ�̣�����Խ���۷е�Խ�ߣ����Ӽ�������Խ��
����⣺��1������ԭ��������8����̬ʱ��������Ų�ʽΪ1s22s22p4���ʴ�Ϊ��1s22s22p4��
��2��ˮ������Oԭ�ӵļ۲������=2+
��6-2��1��=4���Һ���2�Թµ��Ӷԣ����Բ�ȡSP3��ʽ�ӻ���H2O�����е�ԭ����Ϊ3���۵�����Ϊ10��H2S��NH2-��ԭ����Ŀ��Ϊ3���۵�����Ŀ��Ϊ10������ˮ��Ϊ�ȵ����壬�ʴ�Ϊ��sp3��H2S��NH2-��
��3��A��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬��A����
B��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬��B��ȷ��
C����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬��C��ȷ��
D��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬��D��ȷ��
�ʴ�Ϊ��A��
��4�������ڷ��Ӿ��壬�ɾ���ͼ��֪��BΪ�ɱ��ľ���ͼ��������Ϊ���ӣ�CΪ��ľ���ͼ��������Ϊ����ӣ�������ľ���������ͬ����BC���ʴ�Ϊ��BC��
��5��������������51kJ/mol��ˮ���Ӽ仹���ڷ��»�����11kJ/mol��������ͼ��֪��1molˮ�к���2mol�����������=���»���+��������Ա�����������ġ����ܡ���20kJ/mol���ʴ�Ϊ��20��
��6������ɫ����ˮCuSO4�ܽ���H2O�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ����ɴ�������ӵ����ӷ���ʽ��Cu2++4H2O=[Cu��H2O��4]2+��
�ʴ�Ϊ��Cu2++4H2O=[Cu��H2O��4]2+��
��7��������ĵ縺�Բ����������ĵ縺�Բ�縺�ԵIJ�ԽС���伫��Խ�����ʴ�Ϊ��������ĵ縺�Բ����������ĵ縺�Բ
��8���⻯�������ԭ�Ӱ뾶Խ����Խ��������Խ�ϼ����⻯����ԭ�Ӽ�����ԽԶ������Խ�Գƣ����ʵ��۷е�Խ�ͣ����Ӽ�����Խ����
�ʴ�Ϊ�������⻯�������ԭ�Ӱ뾶Խ����Խ�����̣�������Խ�ף��ѣ��ϼ��������⻯����ԭ�Ӽ�����ԽԶ������Խ�Գƣ����Ӽ�����Խ����
��2��ˮ������Oԭ�ӵļ۲������=2+
| 1 |
| 2 |
��3��A��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬��A����
B��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬��B��ȷ��
C����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬��C��ȷ��
D��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬��D��ȷ��
�ʴ�Ϊ��A��
��4�������ڷ��Ӿ��壬�ɾ���ͼ��֪��BΪ�ɱ��ľ���ͼ��������Ϊ���ӣ�CΪ��ľ���ͼ��������Ϊ����ӣ�������ľ���������ͬ����BC���ʴ�Ϊ��BC��
��5��������������51kJ/mol��ˮ���Ӽ仹���ڷ��»�����11kJ/mol��������ͼ��֪��1molˮ�к���2mol�����������=���»���+��������Ա�����������ġ����ܡ���20kJ/mol���ʴ�Ϊ��20��
��6������ɫ����ˮCuSO4�ܽ���H2O�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ����ɴ�������ӵ����ӷ���ʽ��Cu2++4H2O=[Cu��H2O��4]2+��
�ʴ�Ϊ��Cu2++4H2O=[Cu��H2O��4]2+��
��7��������ĵ縺�Բ����������ĵ縺�Բ�縺�ԵIJ�ԽС���伫��Խ�����ʴ�Ϊ��������ĵ縺�Բ����������ĵ縺�Բ
��8���⻯�������ԭ�Ӱ뾶Խ����Խ��������Խ�ϼ����⻯����ԭ�Ӽ�����ԽԶ������Խ�Գƣ����ʵ��۷е�Խ�ͣ����Ӽ�����Խ����
�ʴ�Ϊ�������⻯�������ԭ�Ӱ뾶Խ����Խ�����̣�������Խ�ף��ѣ��ϼ��������⻯����ԭ�Ӽ�����ԽԶ������Խ�Գƣ����Ӽ�����Խ����
���������⿼���˾����ļ��㡢�����Ų�ͼ����д��֪ʶ�㣬�ѵ��Ǽ�������ļ��ܣ������þ�̯�����㾧���ǽⱾ��Ĺؼ����ѶȽϴ�

��ϰ��ϵ�д�
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
�����Ŀ
