��Ŀ����
�ۺ��Ȼ���������һ�ָ�Ч��ˮ���������仯ѧʽΪ[Al2��OH��nCl6-n?XH2O]m�������Ʊ�ԭ���ǵ���AlCl3��Һ��pH��ͨ���ٽ���ˮ����ᾧ��������ͼ��һ�ֹ�ҵ�Ʊ��ۺ��Ȼ�������Ĺ������̣��Ʊ�ԭ����Ҫ�����ӹ���ҵ�ķ���--���ң���Ҫ��Al2O3��Al������SiO2�����ʣ�
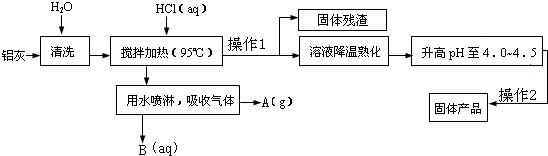
��1��������Ȳ��������з�����Ӧ�����ӷ���ʽ______��______��
��2�����������в���1�Ͳ���2������Ϊ______��
��3����Ӧ�и���ƷA��______�����������п�ѭ��ʹ�õ�������______�������û�ѧʽ��ʾ��
��4��������Ҫ�ϸ������Һ��pH����С�����Ȼ���ˮ�ⲻ��ȫ����mֵƫС����Ʒ���������ή�ͣ���pH������Ҳ�ή�ͣ���ԭ����______��
��5����֪��2Al��s��+
O2��g��=Al2O3��s������H=-akJ?mol-1
3Fe��s��+2O2��g��=Fe3O4��s������H=-bkJ?mol-1
д��Al��Fe3O4�������ȷ�Ӧ���Ȼ�ѧ����ʽ����Hֵ��a��b��ʾ��______��

��1��������Ȳ��������з�����Ӧ�����ӷ���ʽ______��______��
��2�����������в���1�Ͳ���2������Ϊ______��
��3����Ӧ�и���ƷA��______�����������п�ѭ��ʹ�õ�������______�������û�ѧʽ��ʾ��
��4��������Ҫ�ϸ������Һ��pH����С�����Ȼ���ˮ�ⲻ��ȫ����mֵƫС����Ʒ���������ή�ͣ���pH������Ҳ�ή�ͣ���ԭ����______��
��5����֪��2Al��s��+
| 3 |
| 2 |
3Fe��s��+2O2��g��=Fe3O4��s������H=-bkJ?mol-1
д��Al��Fe3O4�������ȷ�Ӧ���Ȼ�ѧ����ʽ����Hֵ��a��b��ʾ��______��
��1�������������ᷴӦ�����Ȼ�����ˮ����Ӧ���ӷ���ʽΪAl2O3+6H+=2Al3++3H2O��
�������ᷴӦ�����Ȼ�������������Ӧ���ӷ���ʽΪ2Al+6H+=2Al3++3H2����
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��2Al+6H+=2Al3++3H2����
��2����������������ᷴӦ�����˺��ռ���Һ������Ũ�������ͣ���pHֵ���Ծ��ã������ռ����������յ����������������Ҫ�ľ��壬�������������в�����͢�ľ�Ϊ���ˣ�
�ʴ�Ϊ�����ˣ�
��3������AΪ�������ᷴӦ���ɵ�������95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq�����ɽ���ѭ��ʹ�ã�
�ʴ�Ϊ��H2��HCl��
��4��������ˮ�⣬Al3++3H2O?Al��OH��3+3H+������������Ũ�ȴٽ�������ˮ�⣬�����ھۺ��Ȼ�������������pH����ʱ���ɽ����ܽ�Ϊ[Al��OH��4]-��
�ʴ�Ϊ��AlCl3��ȫˮ��ΪAl��OH��3��pH����ʱ���ɽ����ܽ�Ϊ[Al��OH��4]-��
��5����2Al��s��+
O2��g��=Al2O3��s������H=-akJ?mol-1
��3Fe��s��+2O2��g��=Fe3O4��s������H=-bkJ?mol-1
���ݸ�˹���ɼ���١�4-�ڡ�3�õ�Al��Fe3O4�������ȷ�Ӧ���Ȼ�ѧ����ʽ��8Al��s��+3Fe3O4��s��=4Al2O3��s��+9Fe��s������H=-4a+3bkJ?mol-1��
�ʴ�Ϊ��8Al��s��+3Fe3O4��s��=4Al2O3��s��+9Fe��s������H=-4a+3bkJ?mol-1��
�������ᷴӦ�����Ȼ�������������Ӧ���ӷ���ʽΪ2Al+6H+=2Al3++3H2����
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O��2Al+6H+=2Al3++3H2����
��2����������������ᷴӦ�����˺��ռ���Һ������Ũ�������ͣ���pHֵ���Ծ��ã������ռ����������յ����������������Ҫ�ľ��壬�������������в�����͢�ľ�Ϊ���ˣ�
�ʴ�Ϊ�����ˣ�
��3������AΪ�������ᷴӦ���ɵ�������95��C���ȵ�ʱ��HCl��ӷ�����ˮ���ܾͿ�������HCl���õ�HCl��aq�����ɽ���ѭ��ʹ�ã�
�ʴ�Ϊ��H2��HCl��
��4��������ˮ�⣬Al3++3H2O?Al��OH��3+3H+������������Ũ�ȴٽ�������ˮ�⣬�����ھۺ��Ȼ�������������pH����ʱ���ɽ����ܽ�Ϊ[Al��OH��4]-��
�ʴ�Ϊ��AlCl3��ȫˮ��ΪAl��OH��3��pH����ʱ���ɽ����ܽ�Ϊ[Al��OH��4]-��
��5����2Al��s��+
| 3 |
| 2 |
��3Fe��s��+2O2��g��=Fe3O4��s������H=-bkJ?mol-1
���ݸ�˹���ɼ���١�4-�ڡ�3�õ�Al��Fe3O4�������ȷ�Ӧ���Ȼ�ѧ����ʽ��8Al��s��+3Fe3O4��s��=4Al2O3��s��+9Fe��s������H=-4a+3bkJ?mol-1��
�ʴ�Ϊ��8Al��s��+3Fe3O4��s��=4Al2O3��s��+9Fe��s������H=-4a+3bkJ?mol-1��

��ϰ��ϵ�д�
�����Ŀ



