��Ŀ����
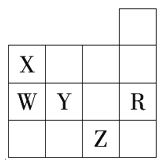
����Ŀ����ͼΪ���ڱ��в���Ԫ�ص�ij������(Xֵ)��ԭ�������仯�Ĺ�ϵ��
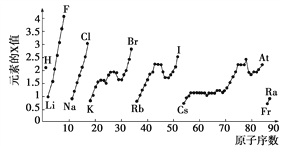
(1)��������ԭ�Ӻ���p����ϵ�������s����ϵ���������ȵ�Ԫ����________(дԪ�ط���)��
(2)ͬ�����ڲ�ͬԪ�ص�Xֵ�仯���ص��� ________________��ͬ�����ڣ�����ԭ������������Xֵ�仯����������________�����ڱ���Xֵ�����ֱ仯�ص�������Ԫ�����ʵ�____________�仯���ɡ�
(3)Xֵ��С��Ԫ�ؼ�����Ԫ�����ڱ���________��
a�����½ǡ� b�����Ͻǡ�����c���ֽ��߸���
(4)���й���Ԫ�ظ����ʵ�˵������ȷ����________(ѡ�����)��
a��Xֵ�ɷ�ӳԪ����������ϼ۵ı仯����
b��Xֵ�ɷ�ӳԭ���ڷ������������ӵ�����
c��Xֵ��С����������Ԫ�ؽ����Ժͷǽ����Ե�ǿ��
���𰸡� O��Mg ͬһ���壬���ϵ��£�Xֵ��С ������ ������ a b��c
����������1������p����������6�����ӣ�s����������2�����ӣ���˶�������ԭ�Ӻ���p����ϵ�������s����ϵ���������ȵ�Ԫ��������Ų�����Ϊ1s22s22p4��1s22s22p63s2�����ֱ�ΪO��Mg����2������ͼʾ��ͬ����Ԫ�ش��ϵ��£�Xֵ��С��ͬ����Ԫ�ش����ң�Xֵ������������ڱ���Xֵ�����ֱ仯�ص�������Ԫ�����ʵ������Ա仯���ɡ���3������ͼʾ���ж�Xֵ��С��Ԫ�ؼ�����Ԫ�����ڱ����½ǡ���ѡa����4���������Ϸ�����֪XֵΪԪ�صĵ縺�ԣ��ܹ���ӳԭ���ڷ������������ӵ��������ܺ���Ԫ�ؽ����Ժͷǽ����Ե�ǿ�������ܷ�ӳԪ����������ϼ۵ı仯���ɣ���ѡbc��

����Ŀ������ʵ�������ȷ���ܴﵽ��ӦĿ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȡ2.0gNaOH���� | ���������ϸ���1����ֽ��Ȼ��������������2g���룬����������NaOH���� |
B | ����ϡ���� | �Ƚ�Ũ��������ձ�����������ˮ |
C | ��֤����������ʴ | �����������Թ��У��������û |
D | ������Һ���Ƿ���NH4+ | ȡ������Һ���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������� |
A. A B. B C. C D. D