��Ŀ����
����Ŀ��ij����С��ͬѧ�Բ�ͬ��������������ʴ������ʵ�顣
ʵ����� | �� | �� | �� | �� |
ʵ�� ���� |
|
|
|
|
һ�ܺ�۲죺
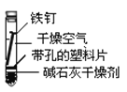
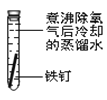
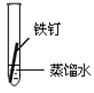
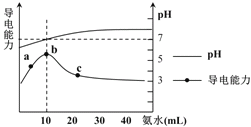
��1����������ʴ�̶�������___����ʵ����ţ���
��2��ʵ�������Ҫ��������____(������ѧ��ʴ�������绯ѧ��ʴ��)��
��3���չ��˵�����δ��ʱϴ�������Ȼ��ƵIJ���Һ�����ڶ������ֺ���ɫ��ߣ���д���йصĵ缫��Ӧʽ�� ����____��
��4����������ʵ�飬����Ϊ�������绯ѧ��ʴ��������____��
��5����������ʾ��ȫ����ÿ����ʴ�����ϵĽ��������൱�����������20%���ϡ�Ϊ������������ʴ�ɲ�ȡ�Ĵ�ʩ��___(�����)��
�ٽ�������ˢ���� �����г���Ȧ�Ƹ� �۽��ֹ��õ�����þ������ �ܽ��ֹ��õ�����̼������
���𰸡��� �绯ѧ��ʴ Fe��2e-=Fe2+ �Ӵ�ˮ�Ϳ��� �٢ڢ�
��������
��1�����ĸ�ʴ�л�ѧ��ʴ�͵绯ѧ��ʴ���֣��绯ѧ��ʴ�Ȼ�ѧ��ʴҪ�죬ע��Ƚ�������������ĸ�ʴ���ͺ�Ӱ�츯ʴ��������
��2��ע������ܷ��γ�ԭ����������жϸ�ʴ�����ͣ�
��3�������Ϸ���������Ӧ������������ԭ��Ӧ��
��4���绯ѧ��ʴ������ԭ��ط�Ӧ����ԭ��ص��γ��������жϣ�
��5�������ķ�����ʩ��������������������������ӵ���������������������е�ơ���ơ�������ȷ���ʹ�����������ˮ�����ʸ��룬�Է�ֹ������ʴ��
��1�����ڸ�����������Ը�ʴ���ڸ�������Ҳ���Ը�ʴ���ܷۢ����绯ѧ��ʴ�������е������Һ����Ũ�ȴ�����ǿ���绯ѧ��ʴ���ʿ죬��ѡ���ܣ�
��2����������ˮ�ܽ��������ܷ����绯ѧ��ʴ��
��3����Ӧ�����Ǹ���������������Ӧ������Fe2��������Ϊ��Fe-2e��=Fe2����
��4���绯ѧ��ʴ������ԭ��ط�Ӧ����ԭ��ص��γ��������жϣ������������Բ�ͬ�ĵ缫���������Һ���պϻ�·��������ʴʱ�����п�������֮���绯ѧ��ʴ�������ǣ����Ӵ������͵������Һ�����볱ʪ�����Ӵ�����
��5���ٽ�������ˢ���ᣬΪ��ӷ���Ĥ�ı����������Ը��������� �����г���Ȧ�Ƹ� ��Ϊ��ӷ���Ĥ�ı����������Ը��������� �۽��ֹ��õ�����þ�����ӣ�����þ�������ã��������������������������� �ܽ��ֹ��õ�����̼�����ӣ������������ӿ��˽����ĸ�ʴ����ѡ�٢ڢۡ�