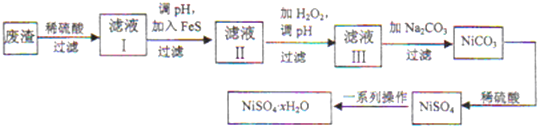
��Ŀ����
4���������ƣ�CaO2����һ�ְ�ȫ�������ʣ������������ȵĽᾧˮ��ͨ����������CaO����֪��2CaO2•nH2O $\frac{\underline{\;\;��\;\;}}{\;}$2CaO+O2��+2nH2O��2CaO2+4HCl�T2CaCl2+2H2O+O2����
�ֳ�ȡ����1.408g����������Ʒ�ֱ��������ʵ�飺
ʵ��һ����һ�ݹ���������Ʒ������ȣ�����õ���O2�ڱ�״�������Ϊ134.4mL��
ʵ���������һ����Ʒ����������ϡ�����У���ַ�Ӧ����������Na2CO3��Һ������õ�1.40g������
��1���Լ���1.408 g��Ʒ��CaO��������
��2���Լ�����Ʒ��CaO2•nH2O��nֵ����д������̣����÷֣���
���� ��1�����1.408g�������Ƽ��ȷ���2CaO2•nH2O $\frac{\underline{\;\;��\;\;}}{\;}$2CaO+O2��+2nH2O��1.40g����Ϊ̼��ƣ����Caԭ���غ��֪CaO2•nH2O�����ʵ�����������������ʵ���������ʽ���㣻
��2��������������ˮ�����������n=$\frac{m}{M}$���㣮
��� �⣺��1��n��O2��=$\frac{0.1344L}{22.4L/mol}$=0.006mol��
һ���У�n��CaO2•nH2O��=2n��O2��=0.012mol��
n��CaCO3��=$\frac{1.40g}{100g/mol}$=0.014mol��
n��CaO��=n��CaCO3��-n��CaO2•nH2O��=0.002mol��
��Ʒ��m��CaO��=0.002mol��56g/mol=0.112g��
����Ʒ��CaO������Ϊ0.112g��
��2��m��H2O��=1.408-0.112-0.012��72=0.432g��
n��H2O��=$\frac{0.432g}{18g/mol}$=0.024 mol��
n��CaO2����n��H2O��=1��n��
���n=2��
����Ʒ��CaO2•nH2O��nֵΪ2��
���� ���⿼�黯ѧ����ʽ�ļ��㣬Ϊ��Ƶ���㣬ע��������ʵ�������ϵ��������غ㼰����ʽ���㣬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14���������ʵ���;��Ҫ�����仯ѧ���ʵ��ǣ�������
| A�� | ͭ�������쵼�� | B�� | �ɱ������˹����� | ||
| C�� | ��Ȼ������ȼ�� | D�� | ʯī�����ɵ�ص缫 |
15�������أ�C15H22O5���Ǵ�ֲ��ƻ��� Ҷ����ȡ�Ŀ���������ű����ҩ��ҹ���ѧ�����������������ؼ�˫�������أ�C15H24O5������Ŀ������о�������2015ŵ��������ѧ��ҽѧ������Ϊ����λ���ŵ���������й�������ѧ�ң�������Ϊ��ɫ��״���壬ζ�࣮ �����ѣ�C2H5-O-C2H5 ���п��ܽ⣬��ˮ�м������ܣ�����˵����ȷ���ǣ�������
| A�� | �����ص�Ħ������Ϊ282 | |
| B�� | ��������ȡ�ƻ��� Ҷ�е������ر���ˮЧ���� | |
| C�� | ������ת��Ϊ˫���������������仯 | |
| D�� | �����صĻ�ѧ�ϳɷ���û���κ��о����� |
12��ij���᳧��������������Ҫ��Fe2O3��FeO������һ������SiO2���������Ʊ�FeCO3�����������£�
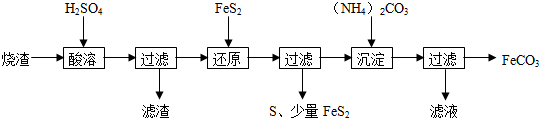
��֪������ԭ��ʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ�����£�
FeS2+14Fe3++8H2O=15Fe2++2SO42-+16H+
��1������FeS2��ԭ����Fe3+�Ƿ�Ӧ��ȫ���Լ�ΪKSCN��Һ��
��2������FeCO3����ϴ�ӣ������Ƿ�ϴ���ķ�����ȡ���һ��ϴ����Һ���μ��Ȼ�����Һ������Һ�Ƿ��г����������������Ѿ�ϴ�Ӹɾ�����������ʱ��pH���˹��ߣ������Ʊ���FeCO3�п��ܻ��е�����������������
��3����д������ԭ��ʱ��Ӧ������ӷ���ʽ��2Fe3++S2-=2Fe2++S����
�ڡ���ԭ��ǰ����Һ�в������ӵ�Ũ�ȼ��±�����Һ����仯���Բ��ƣ���
����㷴Ӧ���б���ԭ��Fe3+�����ʵ���֮�ȣ�д��������̣���

��֪������ԭ��ʱ��FeS2��H2SO4����Ӧ��Fe3+ͨ����Ӧ��ԭ�����з�Ӧ�����£�
FeS2+14Fe3++8H2O=15Fe2++2SO42-+16H+
��1������FeS2��ԭ����Fe3+�Ƿ�Ӧ��ȫ���Լ�ΪKSCN��Һ��
��2������FeCO3����ϴ�ӣ������Ƿ�ϴ���ķ�����ȡ���һ��ϴ����Һ���μ��Ȼ�����Һ������Һ�Ƿ��г����������������Ѿ�ϴ�Ӹɾ�����������ʱ��pH���˹��ߣ������Ʊ���FeCO3�п��ܻ��е�����������������
��3����д������ԭ��ʱ��Ӧ������ӷ���ʽ��2Fe3++S2-=2Fe2++S����
�ڡ���ԭ��ǰ����Һ�в������ӵ�Ũ�ȼ��±�����Һ����仯���Բ��ƣ���
| ���� | ����Ũ�ȣ�mol•L-1�� | |
| ��ԭǰ | ��ԭ�� | |
| Fe2+ | 0.10 | 2.5 |
| SO42- | 3.5 | 3.7 |
19����һ������ˮ��Һ�����ܺ������������е������֣�K+��NH4+��Cl-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г���������
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.03mol���壻
�����ݼ�����BaCl2��Һ�õ��������4.30g������������ϴ�ӡ������������Ϊ2.33g
�ۺ�����ʵ�飬����Ϊ���½�����ȷ���ǣ�������
��һ�ݼ���AgNO3��Һ�г���������
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.03mol���壻
�����ݼ�����BaCl2��Һ�õ��������4.30g������������ϴ�ӡ������������Ϊ2.33g
�ۺ�����ʵ�飬����Ϊ���½�����ȷ���ǣ�������
| A�� | �û��Һ��һ�����У�K+��NH4+��CO32-��SO42-�����ܺ�Cl- | |
| B�� | �û��Һ��-�����У�NH4+��CO32-��SO42-�����ܺ�K+��Cl- | |
| C�� | �û��Һ��һ�����У�NH4+��CO32-��SO42-��Cl-�����ܺ�K+ | |
| D�� | �û��Һ�У�c��K+����0.1mol/L c��CO32-��=0.1mol/L |
9�����и������ʣ�ǰ�����ڵ���ʣ��������ڷǵ���ʵ��ǣ�������
| A�� | NaCl���� ����ͭ | B�� | �� �������� | ||
| C�� | Һ̬�Ĵ��� ���� | D�� | ���ڵ�KNO3 ������Һ |