��Ŀ����
����Ŀ�����仯�����ڻ�ѧ��ҵ���������;����ش��������⣺
��1�����⻯��NaBH4�������Ҫ�����
��NaBH4��BԪ�صĻ��ϼ�Ϊ______��
![]() ��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��NaBH4��ˮ��Ӧ����NaBO2��H2���÷�Ӧ���ɵ����������뻹ԭ��������ʵ���֮��Ϊ______��
��2����ҵ���������![]() ��Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������
��Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������![]() ��FeO��CaO��
��FeO��CaO��![]() ��
��![]() ��
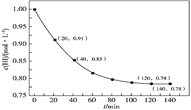
��![]() Ϊԭ���Ʊ�����B�Ĺ���������ͼ��ʾ��
Ϊԭ���Ʊ�����B�Ĺ���������ͼ��ʾ��

��֪��
�������� | Fe3+ | Al3+ |
��ʼ������pH | 2.7 | 3.1 |
������ȫ��pH | 3.7 | 4.9 |
![]() ��������ʱ���������ʯ�����Ŀ��Ϊ______��
��������ʱ���������ʯ�����Ŀ��Ϊ______��
![]() ����1����Ҫ�ɷ�Ϊ______��
����1����Ҫ�ɷ�Ϊ______��
![]() ���������ӡ�ʱ���ȼ�H2O2��Һ����Ŀ��Ϊ______��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����______��
���������ӡ�ʱ���ȼ�H2O2��Һ����Ŀ��Ϊ______��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����______��
![]() �ƵõĴ�����һ��������������BI3��BI3���ȷֽ���Եõ������ĵ������ֽ�
�ƵõĴ�����һ��������������BI3��BI3���ȷֽ���Եõ������ĵ������ֽ�![]() �����Ƴɵ�BI3��ȫ�ֽ⣬���ɵ�
�����Ƴɵ�BI3��ȫ�ֽ⣬���ɵ�![]() ��0.30mol��L��1Na2S2O3��Һ�ζ�
��0.30mol��L��1Na2S2O3��Һ�ζ�![]() ���յ㣬����18.00mL Na2S2O3��Һ��ʢװNa2S2O3��ҺӦ��______
���յ㣬����18.00mL Na2S2O3��Һ��ʢװNa2S2O3��ҺӦ��______![]() ���ʽ����ʽ��
���ʽ����ʽ��![]() �ζ��ܣ��ô�����Ʒ�Ĵ���Ϊ______��
�ζ��ܣ��ô�����Ʒ�Ĵ���Ϊ______��
���𰸡�+3 4NaH+B(OCH3)3��NaBH4+3CH3ONa 1:1 ����Ӵ�������ӿ췴Ӧ���� SiO2��CaSO4 �����е�![]() ����Ϊ
����Ϊ![]() ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ ��ʽ 97.2%
ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ ��ʽ 97.2%
��������
��1����NaBH4��Na��+1�ۣ�H�ǣ�1�ۣ���BԪ�صĻ��ϼۣ����ݻ��ϼ۴�����Ϊ0���ó�BΪ+3�ۡ�
![]() ��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ4NaH+B(OCH3)3��NaBH4+3CH3ONa ��
��ҵ�Ͽ������������B(OCH3)3���⻯��NaH��Ӧ�Ʊ�NaBH4����Ӧ����һ�ֲ���Ϊ�״��ƣ�CH3ONa�����÷�Ӧ�Ļ�ѧ����ʽΪ4NaH+B(OCH3)3��NaBH4+3CH3ONa ��
��NaBH4��ˮ��Ӧ����NaBO2��H2��NaBH4��2H2O��NaBO2��4H2����NaBH4��H���������õ��������ˮ���ⱻ��ԭ���õ���ԭ����÷�Ӧ���ɵ����������뻹ԭ��������ʵ���֮��Ϊ1:1��
��2�����������Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4����������Fe2O3��FeO��CaO��Al2O3��SiO2�ȣ�Ϊԭ���Ʊ����ᣨH3BO3���������̿�֪���������ܽ�ֻ��SiO2���ܣ�Mg2B2O5��H2O+2H2SO4��2H3BO3+2MgSO4��CaOת��Ϊ����ˮ��CaSO4�����������ӡ����ȼ�H2O2��Һ������������ת��Ϊ�����ӣ�������Һ��pHԼΪ5��ʹ�����ӡ������Ӿ�ת��Ϊ������������Ϊ��������������������Ȼ������Ũ������ȴ�ᾧ�����˷����H3BO3��
�١�������ʱ���������ʯ�����Ŀ��Ϊ����Ӵ�������ӿ췴Ӧ���ʡ�
�ڼ������ܽ�ֻ��SiO2���ܣ�Mg2B2O5��H2O+2H2SO4��2H3BO3+2MgSO4��CaOת��Ϊ����ˮ��CaSO4������Fe3O4�Ĵ��ԣ��ɽ���ӡ��������з��롣���������л�ʣ���������SiO2��CaSO4������1����Ҫ�ɷ�ΪSiO2��CaSO4��
�ۡ��������ӡ����ȼ�H2O2��Һ����Ŀ��Ϊ�����е�![]() ����Ϊ
����Ϊ![]() ��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ��
��Ȼ���ٵ�����Һ��pH��5.0��Ŀ����ʹFe3��ת��ΪFe(OH)3������Al3��ת��ΪAl(OH)3��������ȥ��
��Na2S2O3��Һ�ʼ��ԣ�Ӧ���ڼ�ʽ�ζ����У���������Ƶ����ʵ���Ϊ��0.30mol��L��1��0.018L=0.0054mol�����ݹ�ϵʽ��B��BI3��![]() I2��3S2O32������n��B��=
I2��3S2O32������n��B��=![]() n��S2O32����=0.0018mol���������Ϊ��10.81g��mol��1��0.0018mol=0.01944g����������ĺ���Ϊ��
n��S2O32����=0.0018mol���������Ϊ��10.81g��mol��1��0.0018mol=0.01944g����������ĺ���Ϊ��![]() ��100%=97.2%��
��100%=97.2%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����֪��Ӧ![]()
![]() ����ʼ������
����ʼ������![]() Ϊ
Ϊ![]() ��
��![]() Ϊ
Ϊ![]() ��HCOOHΪ
��HCOOHΪ![]() ����һ���¶��£���ͬʱ�̼������������ʵ������£�
����һ���¶��£���ͬʱ�̼������������ʵ������£�![]() ע���������ʵ�λ��
ע���������ʵ�λ��![]() ��ʾ
��ʾ![]()
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 |
|
|
|
|
|
|
|
|
|
|
|
|
|
����˵������ȷ���� ![]()
![]()
A.�Ա���![]() Ϊ��λ��
Ϊ��λ��![]() ������ƽ����Ӧ������
������ƽ����Ӧ������![]() ֮��
֮��
B.ǰ![]() ����������ƽ����Ӧ����Ϊ
����������ƽ����Ӧ����Ϊ![]()
C.���������ķ�Ӧ������������С��ԭ���Ǹ÷�Ӧ����
D.��ʼʱ����������NaOH��Ҳ���Լӿ��������ˮ������
����Ŀ����֪��![]()
![]() ����
����![]() ���ܱ������н���ģ��ϳ�ʵ�飬��
���ܱ������н���ģ��ϳ�ʵ�飬��![]() ��
��![]() ͨ�������У��ֱ���
ͨ�������У��ֱ���![]() ��
��![]() ��Ӧ��ÿ��һ��ʱ���������еļ״���Ũ�����£�
��Ӧ��ÿ��һ��ʱ���������еļ״���Ũ�����£�
| 10 | 20 | 30 | 40 | 50 | 60 |
300 | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
500 | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
����˵����ȷ���� ![]()
![]()
A.![]() ʱ����ʼ
ʱ����ʼ![]() ��
��![]() ��ƽ����Ӧ����
��ƽ����Ӧ����![]()
B.��Ӧ�ﵽƽ��ʱ�����¶���CO��![]() ��ת����֮�Ⱦ�Ϊ
��ת����֮�Ⱦ�Ϊ![]()
C.![]() ��Ӧ�ﵽƽ��ʱ���ų�������Ϊ
��Ӧ�ﵽƽ��ʱ���ų�������Ϊ![]()
D.![]() ʱ�����������ݻ�ѹ����ԭ����
ʱ�����������ݻ�ѹ����ԭ����![]() ����
����![]() ����
����![]() ��С
��С