��Ŀ����
����Ŀ����֪25��ʱCH3COOH�ĵ��볣��K=1.6��10-5�����¶�����20ml0.01mol/L CH3COOH��Һ����μ���0.01mol/LKOH��Һ����pH�仯������ͼ��ʾ(�����¶Ⱥ���Һ����ı仯)������˵����ȷ���ǣ� ��
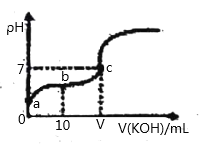
A.a����c(H+)Ϊ4.0��10-5mol/L
B.b����Һ������c(CH3COO-) +c(OH-)=c(CH3COOH)+c(H+)
C.V=20
D.�ζ������У�c(CH3COO-)+c(CH3COOH)+c(K+)=0.01mol/L
���𰸡�D
��������
A. CH3COOH![]() CH3COO����H�������ݵ���ƽ�ⳣ���Ķ��壬K=
CH3COO����H�������ݵ���ƽ�ⳣ���Ķ��壬K=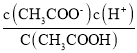 =
= ![]() =1.6��10��5�����c(H��)=4��10��4mol��L��1����A����
=1.6��10��5�����c(H��)=4��10��4mol��L��1����A����
B. ���ݵ���غ㣬c(K��)��c(H��)=c(CH3COO��)��c(OH��) �٣�b��ʱ����KOH10mL����ʱ�����Һ�൱��0.0001mol CH3COOH��0.0001mol CH3COOK��ϣ���2c(K��)= c(CH3COO��)+ c(CH3COOH) �ڣ�����2-�ڵã�c(CH3COOH)+ 2c(H��)= c(CH3COO-)+ 2c(OH-)����B����
C. ��V=20��������� KOHǡ����ȫ��Ӧ������ΪCH3COOK����ʱ��Һ�ʼ��ԣ�c����Һ�����ԣ�����V<20����C����
D. �ζ��������������غ㣬n(CH3COO��)+ n(CH3COOH)=0.02L��0.01mol/L=0.0002mol�������0.01mol/LKOH VL����![]() =
= ![]() = c(CH3COO-)+c(CH3COOH)+c(K+)=0.01mol/L����D��ȷ��
= c(CH3COO-)+c(CH3COOH)+c(K+)=0.01mol/L����D��ȷ��
��ȷ����D��