��Ŀ����
һ���¶��£���һ���ݻ�Ϊ1L���ܱ������У�����1mol H2(g)��1mol I2(g)��������ӦH2(g)+ I2(g)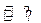 2HI(g)������ַ�Ӧ�ﵽƽ������ɵ�HI(g)�����������50%���ڸ��¶��£�����һ���ݻ�Ϊ2L���ܱ������г���1mol HI(g)������ӦHI(g)
2HI(g)������ַ�Ӧ�ﵽƽ������ɵ�HI(g)�����������50%���ڸ��¶��£�����һ���ݻ�Ϊ2L���ܱ������г���1mol HI(g)������ӦHI(g)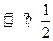 H2(g)+
H2(g)+ 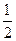 I2(g)���������ж���ȷ����
I2(g)���������ж���ȷ����
 2HI(g)������ַ�Ӧ�ﵽƽ������ɵ�HI(g)�����������50%���ڸ��¶��£�����һ���ݻ�Ϊ2L���ܱ������г���1mol HI(g)������ӦHI(g)
2HI(g)������ַ�Ӧ�ﵽƽ������ɵ�HI(g)�����������50%���ڸ��¶��£�����һ���ݻ�Ϊ2L���ܱ������г���1mol HI(g)������ӦHI(g)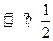 H2(g)+
H2(g)+ 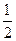 I2(g)���������ж���ȷ����
I2(g)���������ж���ȷ����| A����һ��Ӧ��ƽ�ⳣ��Ϊ1 |
| B����һ��Ӧ��ƽ�ⳣ��Ϊ0.5 |
| C����һ��Ӧ�ﵽƽ��ʱ��H2��ƽ��Ũ��Ϊ0.25mol��L��1 |
| D����һ��Ӧ�ﵽƽ��ʱ��HI(g)��ƽ��Ũ��Ϊ0.5 mol��L��1 |
B
��

��ϰ��ϵ�д�
�����Ŀ
 ��N2O4��g������ƽ���NO2��ת����Ϊ��1�����������г���2 mol NO2���ٴδﵽƽ�⣬NO2��ת����Ϊ��2�����������г���1 mol N2O4���ٴδﵽƽ�⣬NO2��ת����Ϊ��3 �����1���3�Ĺ�ϵ��ȷ���ǣ� ��
��N2O4��g������ƽ���NO2��ת����Ϊ��1�����������г���2 mol NO2���ٴδﵽƽ�⣬NO2��ת����Ϊ��2�����������г���1 mol N2O4���ٴδﵽƽ�⣬NO2��ת����Ϊ��3 �����1���3�Ĺ�ϵ��ȷ���ǣ� ��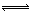 CO2��g����H2��g����K��1��850��ʱ�������ݻ�Ϊ2L���ܱ�������ͬʱ����1.0mol CO��3.0mol H2O��1.0mol CO2��xmol H2��
CO2��g����H2��g����K��1��850��ʱ�������ݻ�Ϊ2L���ܱ�������ͬʱ����1.0mol CO��3.0mol H2O��1.0mol CO2��xmol H2�� CO2(g)ʮH2 (g)����H��0��CO��H2OŨ�ȱ仯����ͼ����0~4min��ƽ����Ӧ���ʦ�(CO)�� mol��(L��min)����ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ ��
CO2(g)ʮH2 (g)����H��0��CO��H2OŨ�ȱ仯����ͼ����0~4min��ƽ����Ӧ���ʦ�(CO)�� mol��(L��min)����ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ ��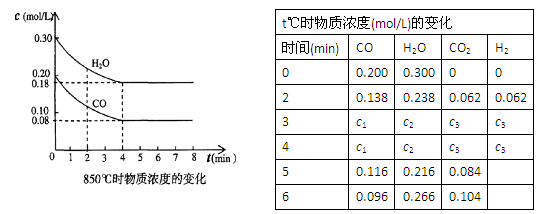
 ״̬��c1 0.08 (�� ������������������)��
״̬��c1 0.08 (�� ������������������)�� 2AB(g)�ﵽƽ��״̬�ı�־�ǣ� ��
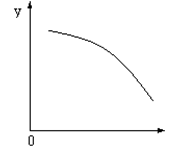
2AB(g)�ﵽƽ��״̬�ı�־�ǣ� �� pC(g)+qD(g)��Ӧ�����У���������������ʱ��C�������������¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ��T2��T1��,����ͼ�����߷������ж�������������ȷ����
pC(g)+qD(g)��Ӧ�����У���������������ʱ��C�������������¶ȣ�T����ѹǿ��P���Ĺ�ϵ��ͼ��T2��T1��,����ͼ�����߷������ж�������������ȷ����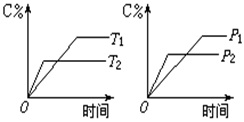
 2C(g)��H<0,Ϊ��ʹƽ��������
2C(g)��H<0,Ϊ��ʹƽ�������� C�ķ����ƶ���Ӧѡ��������ǡ� ��
C�ķ����ƶ���Ӧѡ��������ǡ� �� O2(g)+ 2NO(g)����H��0���ﵽ
O2(g)+ 2NO(g)����H��0���ﵽ ƽ�⡣���ı�����һ������X��Y��X�ı仯����ͼ�����ߵ���
ƽ�⡣���ı�����һ������X��Y��X�ı仯����ͼ�����ߵ���