��Ŀ����
����Ŀ��ʵ��������NaOH��������1.0molL-1��NaOH��Һ230mL��
��1��������Һʱ��һ����Է�Ϊ���¼������裺��������пո�
A.���㣻B.������C.___��D.��ȴ��E.��Һ��F.___��G.���ݣ�H.ҡ�ȡ�װƿ��
��2����ʵ������õ�����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ���Ͳ������___mL����ƿ��ʹ������ƿǰ������е�һ��������___��
��3�������___g�ռ���壬����Ӧ�÷���___�г�����
��4�������ƹ����У���������������ȷ�ģ����в���������Ũ��ƫ�ߵ���___��
A.û��ϴ���ձ��Ͳ�����
B.ת����Һʱ������������������ƿ����
C.����ƿ�����������������ˮ
D.����ʱ���ӿ̶���
E.δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
��5��������������Һȡ��100mL���ٽ���100mL��Һ��ˮϡ�ͳ�1L����Һ����ϡ�ͺ�������Һ�����ʵ���Ũ��Ϊ___��
���𰸡��ܽ� ϴ�� 250mL ��© 10.0 �ձ�������� DE 0.1mol/L
��������
(1)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��ݴ˽��
(2)�������Ʋ���ѡ����Ҫ����������������Һ���ѡ������ƿ�������ƿ���л�����Ϊ��ֹʹ�ù�����©ˮ��ʹ��ǰ��Ҫ����Ƿ�©ˮ��
(3)����m��cVM������Ҫ���ʵ�������������ʴ������Ӧ�ڲ��������н��У�
(4)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c��![]() ������������
������������
(5)������Һϡ�������������ʵ����ʵ���������㡣
(1)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�
(2)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�����������ƽ��ҩ�ס����������ձ�����ͷ�ιܡ���Ͳ������ƿ������1.0molL1��NaOH��Һ230mL��Ӧѡ��250mL����ƿ�����Ի�ȱ��������250mL����ƿ������ƿ���л�����Ϊ��ֹʹ�ù�����©ˮ��ʹ��ǰ��Ҫ����Ƿ�©ˮ��
(3)����1.0molL1��NaOH��Һ230mL��Ӧѡ��250mL����ƿ����Ҫ���ʵ�����m��1.0mol/L��0.25L��40g/mol��10.0g���������ƾ��и�ʴ�ԣ�Ӧ���ձ���������г�����
(4)A��û��ϴ���ձ��Ͳ������������������ʵ���ƫС����ҺŨ��ƫС��A��ѡ��
B��ת����Һʱ������������������ƿ���棬�����������ʵ���ƫС����ҺŨ��ƫС��B��ѡ��
C������ƿ�����������������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬C��ѡ��
D������ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ�Dѡ��
E��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�Eѡ����ѡDE��
(5)��ϡ�ͺ���ҺŨ��Ϊc����������Һϡ�������������ʵ����ʵ�������ã�1.0molL1��100mL��1000mL��c�����c��0.1mol/L��

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�����Ŀ��ũҵ�Ի��ʵ������Ǻϳɰ���ҵ��չ�ij־��ƶ�������һ�ݻ�Ϊ2 L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ��
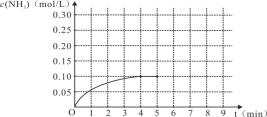
(1)N2(g)��3H2(g)![]() 2NH3(g)����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ������ͼ����ӷ�Ӧ��ʼ��ƽ��ʱ��������ƽ����Ӧ����Ϊ___________________��
2NH3(g)����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ������ͼ����ӷ�Ӧ��ʼ��ƽ��ʱ��������ƽ����Ӧ����Ϊ___________________��
(2)���¶��£���ӦN2(g)+3H2(g)![]() 2NH3(g)+ Q��Q>0����ƽ�ⳣ������ʽΪ__________��
2NH3(g)+ Q��Q>0����ƽ�ⳣ������ʽΪ__________��
��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
T/�� | 25 | 125 | 225 |
ƽ�ⳣ��K | 4��106 | K1 | K2 |
���ж�K1______ K2����д��>����=������<������ԭ����_________________________________
(3)������˵���ϳɰ���Ӧ�Ѵﵽƽ��״̬����________������ĸ������Ӧ���ڹ̶�������ܱ������н��еģ�
a��3v(N2) = v(H2) b�� ![]() �������仯 c�����������ܶȱ��ֲ���
�������仯 c�����������ܶȱ��ֲ���
d��25��ʱ����������� c(NH3)=0.2 mol��L-1�� c(H2) =c(N2) =0.01 mol��L-1
(4) ���������£�NH3����������NO����Ⱦ���������ֶԻ����������ʡ�д����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ��____________���÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ____��
(5) pH��ͬ�İ�ˮ������������Һ���ֱ�������ˮϡ����ԭ�������m����n����ϡ�ͺ�����Һ��pH����ͬ����m________n������>������<������������
����Ŀ����֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ������(������������ȫ������)��

ʵ��ǰ | ʵ��� | |
(�������U�ι�)������ | 101.1g | 102.9g |
(����ʯ��ˮ�����ƿ)������ | 312.0g | 314.2g |
����ʵ��������գ�
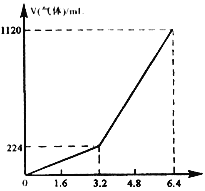
��1��ʵ����Ϻ���������ˮ������Ϊ____g��������ƿ������һ�����Σ�������Ϊ______g��
��2�����ɵ�ˮ����Ԫ�ص�����Ϊ________g��
��3�����ɵ�CO2��̼Ԫ�ص�����Ϊ________g��
��4����ȼ����̼����Ԫ��������Ϊ________��
����Ŀ����(N2H4)��һ��Ӧ�ù㷺�Ļ���ԭ�ϡ���ҵ���Ⱥϳɰ�����N2(g)+3H2(g) ![]() 2NH3(g)+Q(Q��0)���ٽ�һ���Ʊ��¡����������գ�
2NH3(g)+Q(Q��0)���ٽ�һ���Ʊ��¡����������գ�

��1���ϳɰ���Ҫѡ����ʵĴ���,�ֱ�ѡ��A��B��C���ִ�����������,���ý����ͼ��ʾ(����������ͬ)��������������ѡ��Ĵ�����___(�A����B����C��)�������ǣ�________________��
��2��һ�������£������ܱ������н��еĺϳɰ���Ӧ��ƽ���������������ʱ,��ͬʱѹ������������������¶ȴ���ƽ�����ԭƽ����ȣ��뽫�й��������ı仯����������±���(���������С������ȷ����)
��Ӧ���� | ƽ�ⳣ��K | ����������� | |
�仯��� | __ | ____ | ____ |
��3���¿���Ϊ���ȼ�ϣ�4gN2H4(g)��NO2������ȼ�����ɵ�������̬ˮ���ų�71kJ��������д���÷�Ӧ�Ļ�ѧ����ʽ��________��
��4������Ҫ�����������ʺ����ᡣ��ʮ���塱�ڼ䣬Ԥ���ҹ��ϳɰ�����������������������ͼ�Dz�ͬ�¶ȺͲ�ͬѹǿ��,��Ӧ�ﵽƽ���,�������NH3����(���%)�ı仯�������֪��ʼʱn(N2)��n(H2)=1:3���ж�pl��p2ѹǿ�Ĵ�С��ϵ��pl___p2(ѡ���������������=��)��

����Ŀ�������������Ӽ�����1��2-�������顣��ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣

��֪��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
(1)��ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�����_______________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________����ȫƿb�������������Ǣ�_______________��
(2)����c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_____________________��
(3)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________(д����������)��
(4)��ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ (����ĸ)��
A���ؽᾧ B������ C����ȡ D������
(5)ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������______��