��Ŀ����
9�� ijС��ͬѧΪ�Ƚ�����������NO3-��SO42-��Fe3+��������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ��飩��
ijС��ͬѧΪ�Ƚ�����������NO3-��SO42-��Fe3+��������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ��飩��ʵ���¼���£�
| ʵ����� | ʵ������ | |
| �� | ����c��������ϡHNO3����װ��B�У��رջ���c | B��dz��ɫ��Һ������Ϊ����ɫ��һ��ʱ�����Һ���ձ�Ϊ��ɫ�� |
| �� | ��ע����ȡ������Bװ���е���Һ������KSCN��Һ | ��Һ��Ϊ��ɫ�� |
| �� | ����b����Aװ���м����������ᣬ�رջ���b | A�в������壻B�������ݣ�Һ��������������ɫ�������ɣ� |
| �� | һ��ʱ�����ע����ȡ������Bװ���е���Һ���� | �� |
| �� | ����a��ͨ��һ��ʱ����� | ---- |
��1������Fe��NO3��2��Һʱ�����������ۣ�Ŀ���ǣ��û�ѧ����ʽ��ʾ��2Fe��NO3��3+Fe�T3Fe��NO3��2��
��2��ʵ����У�������Ӧ�����ӷ���ʽ��3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O��
��3�����ϱ�����Fe2+����NO����γ�����ɫ����[Fe��NO��]2+��Fe2++NO?[Fe��NO��]2+
��ƽ���ƶ�ԭ������ʵ��I����Һ������ɫ��Ϊ��ɫ��ԭ����Fe2+��ϡ��������ΪFe3+��ʹ��Һ��c��Fe2+�����½���ͬʱNO�����ݳ���Ҳʹ��Һ��c��NO�����½�����ƽ��Fe2++NO?[Fe��NO��]2+���ƣ���Һ��ɫ��ȥ����ʾ��Fe3+�Ļ�ɫ����
��4������ʵ������ͬѧ�ǵó��˽��ۣ���
��ʵ����ĺ��������Ǽ���K3[Fe��CN��6]��Һ���۲쵽��������������ɫ������
����ʵ��ó��Ľ����������ԣ�NO3-��Fe3+��SO42-��
��5��ʵ�鷴˼
��ʵ�����V��Ŀ���ǽ�װ���е�SO2��NO��NO2�������Ž�NaOH��Һ�����գ���ֹ��Ⱦ������
��ʵ�������ʼʱB����Һ����ɫ�������Ա仯����ʱ��Һ�з�����Ӧ�����ӷ���ʽ��3SO2+2NO3-+2H2O�T3SO42-+2NO��+4H+��
����ͬѧ��Ϊװ���еĿ��������ʵ����۵ĵó���Ӧ��ʵ��ǰͨһ��ʱ��ĵ��������Ƿ�ͬ��ÿ����������Dz�ͬ�⣬
a��ʵ�������Һ����ɫ����NO���ɣ�˵����������Fe2+��������NO3-��Fe3+��
b��ʵ�������Һ�м��Fe2+��˵����������ԭ��Fe3+��������Fe3+��SO42-��
��װ�����Ƿ��������أ���
���� ��1�����������ױ������е���������Ϊ�����������������Ի�ԭ������Ϊ����������
��2������c��������ϡHNO3����װ��B�У��رջ���c��B��dz��ɫ��Һ������Ϊ����ɫ��һ��ʱ�����Һ���ձ�Ϊ��ɫ���������ӱ�����Ϊ�����ӣ�
��3��Fe2+����NO����γ�����ɫ����[Fe��NO��]2+��Fe2++NO?[Fe��NO��]2+���������ӱ�������ƽ��������У���������ҺΪ��ɫ��Һ��
��4���ٴ���b����Aװ���м����������ᣬ�رջ���b��A�в������壻B�������ݣ�Һ��������������ɫ�������ɣ�һ��ʱ�����ע����ȡ������Bװ���е���ҺΪ�������ӵ���Һ������K3[Fe��CN��6]��Һ������ɫ������
��������ԭ��Ӧ�е��������������Դ����������������
��5���ٴ���a��ͨ��һ��ʱ������ǽ�װ���е�SO2��NO��NO2�������Ž�NaOH��Һ�����գ�
��ʵ���������b����Aװ���м����������ᣬ�رջ���b����ʼʱB����Һ����ɫ�������Ա仯��ϡ����������������ķ�Ӧ���������һ���������壻
��ʵ��������B��dz��ɫ��Һ������Ϊ����ɫ��һ��ʱ�����Һ���ձ�Ϊ��ɫ������������������Ϊ�����ӣ�ʵ�������Һ�м��Fe2+��˵����������ԭ��Fe3+��������е������أ�
��� �⣺��1�����������ױ������е���������Ϊ��������Ŀ���Ǽ��������Ի�ԭ������Ϊ������������Ӧ�Ļ�ѧ����ʽΪ��2Fe��NO3��3+Fe�T3Fe��NO3��2��
�ʴ�Ϊ��2Fe��NO3��3+Fe�T3Fe��NO3��2��
��2������c��������ϡHNO3����װ��B�У��رջ���c��B��dz��ɫ��Һ������Ϊ����ɫ��һ��ʱ�����Һ���ձ�Ϊ��ɫ���������ӱ�����Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ��3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O��
�ʴ�Ϊ��3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O��
��3��Fe2+����NO����γ�����ɫ����[Fe��NO��]2+��Fe2++NO?[Fe��NO��]2+���������ӱ�������ƽ��������У���Һ��ɫ��ȥ����ʾ��Fe3+�Ļ�ɫ��
�ʴ�Ϊ��Fe2+��ϡ��������ΪFe3+��ʹ��Һ��c��Fe2+�����½���ͬʱNO�����ݳ���Ҳʹ��Һ��c��NO�����½�����ƽ��Fe2++NO?[Fe��NO��]2+���ƣ���Һ��ɫ��ȥ����ʾ��Fe3+�Ļ�ɫ��
��4���ٴ���b����Aװ���м����������ᣬ�رջ���b��A�в������壻B�������ݣ�Һ��������������ɫ�������ɣ�һ��ʱ�����ע����ȡ������Bװ���е���ҺΪ�������ӵ���Һ������K3[Fe��CN��6]��Һ������ɫ������
�ʴ�Ϊ������K3[Fe��CN��6]��Һ��������ɫ������
��ʵ��������������������ӣ�˵�������Ӱ������������������ӣ�ʵ���������b����Aװ���м����������ᣬ�رջ���b����ʼʱB����Һ����ɫ�������Ա仯��ϡ����������������ķ�Ӧ���������һ���������壬˵�����������Դ������������ᣬʵ����Ƕ�������ͨ��������Һ����������ԭ��Ӧ�������ӵ�Ӱ����������������ᣬ�����Դ�СΪ��NO3-��Fe3+��SO42-��
�ʴ�Ϊ�������ԣ�NO3-��Fe3+��SO42-��
��5���ٴ���a��ͨ��һ��ʱ���������װ���е�SO2��NO��NO2�������Ž�NaOH��Һ�����գ���ֹ��Ⱦ������
�ʴ�Ϊ����װ���е�SO2��NO��NO2�������Ž�NaOH��Һ�����գ���ֹ��Ⱦ������
��ʵ��������ɵĶ�������ͨ��װ��B��Һ������������������������������ᣬ��Ӧ������ɫ����һ����������Ӧ�����ӷ���ʽΪ��3SO2+2NO3-+2H2O�T3SO42-+2NO��+4H+��
�ʴ�Ϊ��3SO2+2NO3-+2H2O�T3SO42-+2NO��+4H+��
��ʵ��������B��dz��ɫ��Һ������Ϊ����ɫ��һ��ʱ�����Һ���ձ�Ϊ��ɫ������������������Ϊ�����ӣ�ʵ�������Һ�м��Fe2+��˵����������ԭ��Fe3+������ʵ������������е������أ�װ���еĿ����������ʵ����ۣ�
�ʴ�Ϊ����ͬ�⣬ʵ�������Һ����ɫ����NO���ɣ�˵����������Fe2+��������NO3-��Fe3+��ʵ�������Һ�м��Fe2+��˵����������ԭ��Fe3+��������Fe3+��SO42-����װ�����Ƿ��������أ�
���� ���⿼�����������ʵ�ʵ������жϣ��������ʺ����Ӽ��飬��ѧƽ���������ԭ��Ӧ�ķ���Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | -69.4 kJ/mol | B�� | -45.2kJ/mol | C�� | +69.4 kJ/mol | D�� | +45.2 kJ/mol |
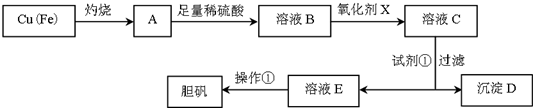
��֪��
| ��Һ�б��������� | Fe3+ | Fe2+ | Cu2+ |
| ��ȫ���������������ʱ����Һ��pH | ��3.7 | ��6.4 | ��4.4 |
��1����ҺB�к��е���������Fe2+��Fe3+��H+��Cu2+�������ӷ��ţ���
��2��������������������������X����b������ĸ����a��NaClO b��H2O2 c��KMnO4
��3�������Լ�����Ϊ�˵���pH���Լ��ٿ���ѡ��CuO��CuCO3��Cu��OH��2���ѧʽ����
��4�������ٵ�����������Ũ������ȴ�ᾧ��
��5������D����������Եõ�FeCl3������FeCl3��Һ��������˵������ȷ����c
a����FeCl3������Һ��μ����ˮ�У����������ȵõ����ɫҺ�壬��Һ���ܲ��������ЧӦ
b����FeCl3��Һ�μ�NaOH��Һ�����ֺ��ɫ����
c����FeCl3��Һ�������ɲ����գ��õ�FeCl3����
d����FeCl3��Һ�еμ�KSCN��Һ����Һ��Ϊ��ɫ
��6������D������������ۣ������Ƶ�FeCl2��Һ��ʵ���ұ���FeCl2��Һ���������������ۣ���ԭ����2Fe3++Fe=3Fe2+�������ӷ���ʽ��ʾ����
| A�� | ���ȵ�����ˮ������Ӧ������������������ | |
| B�� | ����������ͨ�뵽��ɫʯ����Һ�У���Һ�ȱ�����ɫ | |
| C�� | ����ʱ���ɽ��Թܡ�����������ֱ���ھƾ������ϼ��� | |
| D�� | �����������ƹ���ʱ��Ӧ���������ƹ�����ڳ���ֽ�ϳ��� |
| ѡ�� | ���� | ���� |
| A | ��ͬ���ʵ���Ũ����Һ��pH�� Ca��ClO��2��CH3COONa | ���ԣ�CH3COOH��HClO |
| B | ͬ�����ͬpH������ʹ�������ͬ��п ��Ӧ�����ʣ�������� | ���ԣ�HCl��CH3COOH |
| C | ��ͬ�����µķе㣺 H2O��NH3 | �ǽ����ԣ�O��N |
| D | ��ͬ���ʵ����õ����ӵ���Ŀ�� ϡ���Ũ���� | �����ԣ�ϡ���Ũ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��˵���������ӣ���������ʵ��۵����ͣ��ܶ������� | |
| B�� | ��������ʵĽ����Ժ�ǿ�������������������������ȷ�����Ӧ | |
| C�� | ̼��祿���ʱ���ֽܷ�Ϊ������̼������� | |
| D�� | ��ˮ����蘆Ļ�ѧʽΪCs2SO4������������ˮ |
| A�� | $\frac{3n}{2a}$ mol/L | B�� | $\frac{2n}{3a}$ mol/L | C�� | $\frac{2n}{a}$mol/L | D�� | $\frac{3n}{a}$mol/L |
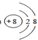 ��
��