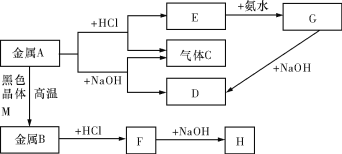
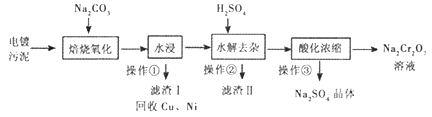
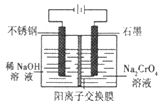
��Ŀ����
����Ŀ��Ϊ̽�����������ֳ������л�������ʣ�ijͬѧ�������ʵ�飬�������ĿҪ����д���пո�
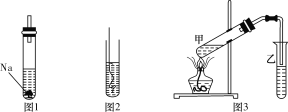
��1������ͼ1��ʾ���Թ���װ�Ҵ�����������Ϊ__________������������
��2������ͼ2��ʾ���Ѽ��ȵ�ͭ˿���뵽װ���Ҵ����Թ��У��ŵ��д̼�����ζ���÷�Ӧ�в������л���Ϊ___________������������
��3����ʳ������ˮ������Ҫ�ɷ�CaCO3����ˮ�����������ˮ����˵�����������___________̼�������������ǿ������������������
��4������ͼ3��ʾװ�ã����Թ���װ�����ᡢ�Ҵ���Ũ���ᣬ����װ�б���̼������Һ�����Թ��Ϸ����ŵ���������ζ�����ʣ�������Ϊ_____________������������
���𰸡���1������ ��2����ȩ ��3��ǿ�� ��4����������
��������
�����������1��Na���Ҵ���Ӧ�����Ҵ��ƺ���������ͼ1�в�������Ϊ������
��2���Ҵ�����ȵ�Cu����������������ȩ����ȩ���д̼�����ζ����ͼ2�в����ľ��д̼�����ζ��Һ��Ϊ��ȩ��
��3��������̼��Ʒ�Ӧ���ɴ���ơ�������̼��ˮ����ǿ����ȡ����ķ�Ӧԭ����֪��������Ϊ���̼�
��4�����Թ���װ�����ᡢ�Ҵ���Ũ���ᣬ��ͼ3�з���������Ӧ�����ɾ�����ζ��������������װ�б���̼������Һ�����Թ��Ϸ����ŵ���������ζ������Ϊ����������
��Ӧ�P���� | �ϼ�λ�� | ��Ӧ���� | ��ѧ����ʽ |
Na | �� | �û���Ӧ | 2CH3CH2OH+2Na��2CH3CH2ONa + H2�� |
HBr���� | �� | ȡ����Ӧ | CH3CH2OH��HBr |
O2��Cu������ | �٢� | ������Ӧ | 2CH3CH2OH+O2 |
ŨH2SO4��170�� | �ڢ� | ��ȥ��Ӧ | CH3CH2OH |
ŨH2SO4��140�� | �٢� | ȡ����Ӧ | 2CH3CH2OH |
CH3COOH��Ũ������ | �� | ȡ������������Ӧ | CH3COOH+CH3CH2OH |