��Ŀ����
����˵����ȷ����
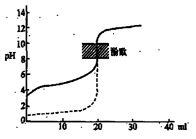

| A����H��0�ķ�Ӧ��������һ�������Է����� |
| B����0.1mol/LNaOH��Һ�ֱ�ζ���ͬ���ʵ���Ũ�Ⱥ���ͬ���������ʹ��ᣬ����ʵ�߱�ʾ���ǵζ���������� |
| C�������£���0.1mol/L��ˮ�У���������NH4Cl���壬��Һ��pH��С |
D�������ܱ������н��еķ�Ӧ3A(g) 2B(g)+C(s)���������������������£��ٳ���һ������A���壬A��ת���ʲ��� 2B(g)+C(s)���������������������£��ٳ���һ������A���壬A��ת���ʲ��� |
C
A���ɹ�ʽ��G=��H��T��S��֪������H��0ʱ���������п����Է����У��ܱ�֤��GС��0���ɣ�������
B������Ϊǿ�ᣬ�������ǿ��pH�ϴ���С������
C�������£���0.1mol/L��ˮ�У�NH3��H2O NH4����OH������������NH4Cl���壬����ͬ����ЧӦ��ƽ�����ƣ�OH��Ũ�ȼ�С����Һ��pH��С����ȷ
NH4����OH������������NH4Cl���壬����ͬ����ЧӦ��ƽ�����ƣ�OH��Ũ�ȼ�С����Һ��pH��С����ȷ
D���ٳ���һ������A���壬�൱�ڼ�ѹ��ƽ��������ϵ����С��CΪ���壩�ķ����ƶ���A��ת��������
�ʴ�ΪC
B������Ϊǿ�ᣬ�������ǿ��pH�ϴ���С������
C�������£���0.1mol/L��ˮ�У�NH3��H2O
 NH4����OH������������NH4Cl���壬����ͬ����ЧӦ��ƽ�����ƣ�OH��Ũ�ȼ�С����Һ��pH��С����ȷ
NH4����OH������������NH4Cl���壬����ͬ����ЧӦ��ƽ�����ƣ�OH��Ũ�ȼ�С����Һ��pH��С����ȷD���ٳ���һ������A���壬�൱�ڼ�ѹ��ƽ��������ϵ����С��CΪ���壩�ķ����ƶ���A��ת��������
�ʴ�ΪC

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 CO2��H2(g) ��Ӧ�����вⶨ�IJ������ݼ��±�������t2��t1��:
CO2��H2(g) ��Ӧ�����вⶨ�IJ������ݼ��±�������t2��t1��: CO(g) +H2(g) ��H= +131.3kJ��mol��1
CO(g) +H2(g) ��H= +131.3kJ��mol��1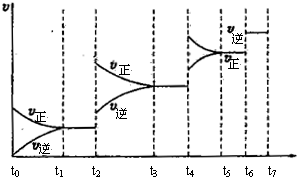
 Z(g)����60s�ﵽƽ�⣬����0.15molZ������˵����ȷ����
Z(g)����60s�ﵽƽ�⣬����0.15molZ������˵����ȷ���� 2NH3��Ӧ�������б����У�����NH3���������ǣ�
2NH3��Ӧ�������б����У�����NH3���������ǣ�

 �����£�Cr(OH)3���ܶȻ�Ksp=10-32����c(Cr3+)����10-5mol/L����Ϊc(Cr3+)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pHֵ����4����ͨ������˵��Cr3+�Ƿ������ȫ����д��������̣�
�����£�Cr(OH)3���ܶȻ�Ksp=10-32����c(Cr3+)����10-5mol/L����Ϊc(Cr3+)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pHֵ����4����ͨ������˵��Cr3+�Ƿ������ȫ����д��������̣�
 H2��g��+I2��g��������ӦΪ���ȷ�Ӧ��
H2��g��+I2��g��������ӦΪ���ȷ�Ӧ�� pC(g)+qD(g)����H<0����֪m+n>p+q.����Ӧ��A��B��ɵĻ���↑ʼ,���ֱ�����������´ﵽƽ��:��100��,1��106Paʱ,A��ת����Ϊ��1����200��,1��106Paʱ,A��ת����Ϊ��2����100��,1��107Paʱ,A��ת����Ϊ��3�����1,��2,��3֮��Ĵ�С��ϵ��
pC(g)+qD(g)����H<0����֪m+n>p+q.����Ӧ��A��B��ɵĻ���↑ʼ,���ֱ�����������´ﵽƽ��:��100��,1��106Paʱ,A��ת����Ϊ��1����200��,1��106Paʱ,A��ת����Ϊ��2����100��,1��107Paʱ,A��ת����Ϊ��3�����1,��2,��3֮��Ĵ�С��ϵ��