��Ŀ����
����ѧ����ѡ��5���л���ѧ������ ��15�֣�
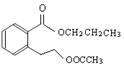
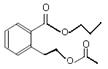
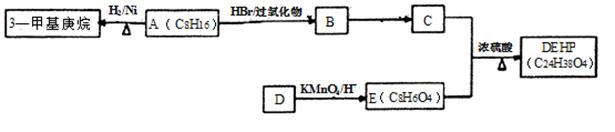
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����

������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���ܷ����ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
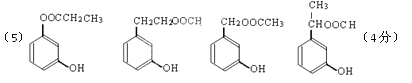
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����

������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���ܷ����ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
��1���Ȼ� ��2�֣�
e ��2�֣�
��2��CH3COOH+CH3CH2CH2OH
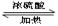 CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣���3���ټӿ췴Ӧ����
 �ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�
��������������п�֪��C�ܸ�NaHCO3������Ӧ������C�����Ȼ����ٴ�C��D�ķ�Ӧ�������Կ���������һ��������Ӧ�����DΪ��������Ϊ�ú�D����Է���������ȣ�����D���ִ�Ϊ��C��һ��̼ԭ�ӵĴ�����EΪ��֧���Ļ�������������ʹ�����ֱ���ġ�
E�е�̼ԭ����ĿΪ��

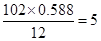 ����ԭ����Ϊ��
����ԭ����Ϊ��
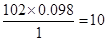 �����ʽΪ��C5H10O2
�����ʽΪ��C5H10O2Ҳ����˵EΪ������������ǿɵ����½⣺
��1���Ȼ� e
��2��CH3COOH+CH3CH2CH2OH
 CH3COOCH2CH2CH3+H2O��2�֣�
CH3COOCH2CH2CH3+H2O��2�֣���3���ټӿ췴Ӧ����
�ڽ������������������ʹƽ����������������ķ����ƶ���3�֣�



��ϰ��ϵ�д�
�����Ŀ
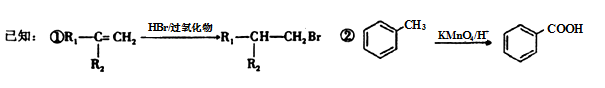

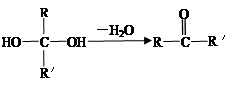
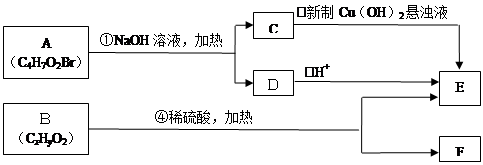
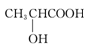 ��������
��������