��Ŀ����
��15�֣�����ѧ����ѡ��5���л���ѧ������
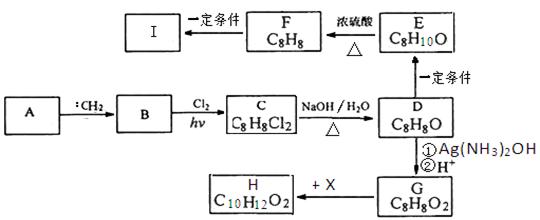
��֪����һ��̼ԭ�������������ǻ�ʱ����������ת������R��R�������������ԭ�ӣ���
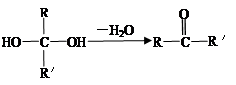
��ͬһ��̼ԭ������������˫���Ľṹ���ȶ���������ͼ�ش��й����⣺
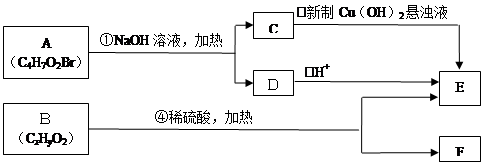
��1��E�к��еĹ����ŵ������� ��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��A�Ľṹ��ʽΪ ��A���ܷ����ķ�Ӧ�� ������ĸ����
a��ȡ����Ӧ b����ȥ��Ӧ c��������Ӧ d����ԭ��Ӧ
��3����֪B����Է�������Ϊ188������ȼ�յIJ�����n��CO2����n��H2O����2��1����B�ķ���ʽΪ ��
��4��F���������ص㣺������FeCl3��Һ������ɫ��Ӧ���ں˴Ź�����������ʾ���������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣��ܳ������⣬������������״�ṹ��д���������������������ȶ��ṹ��F���ܵĽṹ��ʽ�� ��
��֪����һ��̼ԭ�������������ǻ�ʱ����������ת������R��R�������������ԭ�ӣ���
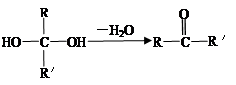
��ͬһ��̼ԭ������������˫���Ľṹ���ȶ���������ͼ�ش��й����⣺
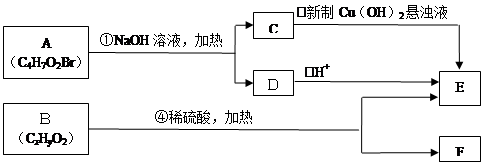
��1��E�к��еĹ����ŵ������� ��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��A�Ľṹ��ʽΪ ��A���ܷ����ķ�Ӧ�� ������ĸ����
a��ȡ����Ӧ b����ȥ��Ӧ c��������Ӧ d����ԭ��Ӧ
��3����֪B����Է�������Ϊ188������ȼ�յIJ�����n��CO2����n��H2O����2��1����B�ķ���ʽΪ ��
��4��F���������ص㣺������FeCl3��Һ������ɫ��Ӧ���ں˴Ź�����������ʾ���������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣��ܳ������⣬������������״�ṹ��д���������������������ȶ��ṹ��F���ܵĽṹ��ʽ�� ��
��15�֣�
��1���Ȼ���2�֣� CH3CHO+2Cu��OH��2 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O
��3�֣�
��2��CH3COOCHBrCH3��2�֣� cd��2�֣�
��3��C12H12O2��2�֣�
��4��


 ����4�֣�
����4�֣�
��1���Ȼ���2�֣� CH3CHO+2Cu��OH��2
 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O��3�֣�
��2��CH3COOCHBrCH3��2�֣� cd��2�֣�
��3��C12H12O2��2�֣�
��4��



 ����4�֣�
����4�֣����������C��D����ת��ΪE��˵��C��D�е�Cԭ������ͬ������2��Cԭ�ӣ�A������������Һ��Ӧ����A��B��˵��A����������ˮ�������ʹ���C�д���ȩ����˵��C�е��ǻ��Ƿ�������֪�ٵķ�Ӧ��2���ǻ�����ͬһ��Cԭ���ϣ�����A�е�Brԭ���������-C-O�е�C������Aˮ������ɵĴ��Զ���ˮ������CΪ��ȩ����DΪ�����ƣ�E�����ᡣ��1�����ݷ���E�д����Ȼ���C������������ͭ��Ӧ�Ļ�ѧ����ʽΪCH3CHO+2Cu��OH��2
 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O��2��A�Ľṹ��ʽΪCH3COOCHBrCH3��A�д�����������ԭ�ӣ����Է���ȡ����Ӧ����ȥ��Ӧ�����Դ�ѡcd��
��3��B����Է���������188����������2��Oԭ�ӣ�����ʣ���C��H����Է���������188-32=156������ȼ�ղ����ж�C:H=1��1�����á����෨������C��H�ĸ�������12������B�ķ���ʽΪC12H12O2��
��4��B������Ӧ���������F����B������������B�ķ���ʽ�ж�B�IJ����Ͷ�Ϊ7��˵������������������ڲ����ͼ�����̼̼������2��̼̼˫����F����10��Cԭ�ӣ�����F���ص��ж�F���б��������ǻ���̼̼������2��̼̼˫���������ϵ�һ�ȴ���ֻ�����֣�˵����������ԭ����2�֣����ǻ���1�֣���������ȡ��������ԭ����2�֡���Fȡ������̼̼��������F����2����λȡ��������F�Ľṹ��ʽΪ

 ����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ
����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ


��ϰ��ϵ�д�
�����Ŀ
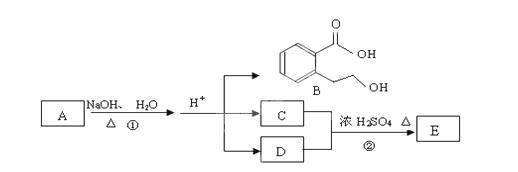
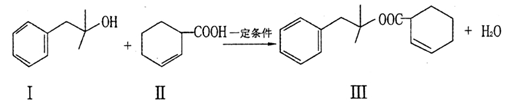
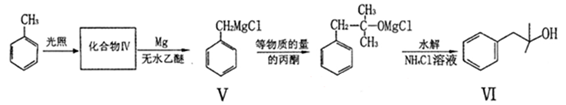
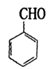 Ҳ�ܷ�������V
Ҳ�ܷ�������V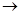 ���ķ�Ӧ����д�������ɴ��Ľṹ��ʽ ��
���ķ�Ӧ����д�������ɴ��Ľṹ��ʽ ��
 �����а����ױ�������
�����а����ױ�������