��Ŀ����
��֪ij��Һ��ֻ����OH-��H+�� ��Cl-�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
��Cl-�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
��c��Cl-����c�� ����c��H+����c��OH-����c��Cl-����c��
����c��H+����c��OH-����c��Cl-����c�� ����c��OH-����c��H+��
����c��OH-����c��H+��
��c�� ����c��Cl-����c��OH-����c��H+����c��Cl-����c��H+����c��
����c��Cl-����c��OH-����c��H+����c��Cl-����c��H+����c�� ����c��OH-��
����c��OH-��
��д���пհף�
��1������Һ��ֻ�ܽ���һ�����ʣ������Һ��_______________�������������ӵ�Ũ�ȵĴ�С˳��Ϊ_______________������ţ���
��2����������ϵ�Т�����ȷ�ģ�����Һ�е�����Ϊ_______________����������ϵ�Т�����ȷ�ģ�����Һ�е�������_______________��
��3��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc��HCl��_________c��NH3��H2O�������С����С�ڡ����ڡ�����
 ��Cl-�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ��
��Cl-�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳�����������ֹ�ϵ����c��Cl-����c��
 ����c��H+����c��OH-����c��Cl-����c��
����c��H+����c��OH-����c��Cl-����c�� ����c��OH-����c��H+��
����c��OH-����c��H+����c��
 ����c��Cl-����c��OH-����c��H+����c��Cl-����c��H+����c��
����c��Cl-����c��OH-����c��H+����c��Cl-����c��H+����c�� ����c��OH-��
����c��OH-����д���пհף�
��1������Һ��ֻ�ܽ���һ�����ʣ������Һ��_______________�������������ӵ�Ũ�ȵĴ�С˳��Ϊ_______________������ţ���
��2����������ϵ�Т�����ȷ�ģ�����Һ�е�����Ϊ_______________����������ϵ�Т�����ȷ�ģ�����Һ�е�������_______________��
��3��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc��HCl��_________c��NH3��H2O�������С����С�ڡ����ڡ�����
��1��NH4Cl�٣�2��NH4Cl��NH3��H2ONH4Cl��HCl(3)С��
��������������������Ͽɵ�NH4Cl��NH3��H2O��HCl�������ʡ�
��1������Һֻ�ܽ�һ�����ʣ�ֻ��NH4Cl��Ϊ����ʱ����Һ�в�ͬʱ���������������ӡ��� ��������ˮ�ⷴӦ
��������ˮ�ⷴӦ +H2O
+H2O NH3��H2O+H+��c��
NH3��H2O+H+��c�� ����С��c��H+����c��OH-��������Һ��c��Cl-����c��
����С��c��H+����c��OH-��������Һ��c��Cl-����c��
 ����c��H+����c��OH-����
����c��H+����c��OH-����
��2������Һ�д���c�� ����c��Cl-����c��OH-����c��H+������c��OH-����c��H+����֪��Һ�ʼ��ԣ�����Һ����NH4Cl��NH3��H2O��϶��ã�������ΪNH4Cl��NH3��H2O������Һ�д���c��Cl-����c��H+����c��
����c��Cl-����c��OH-����c��H+������c��OH-����c��H+����֪��Һ�ʼ��ԣ�����Һ����NH4Cl��NH3��H2O��϶��ã�������ΪNH4Cl��NH3��H2O������Һ�д���c��Cl-����c��H+����c�� ����c��OH-������c��H+����c��
����c��OH-������c��H+����c�� ����֪��Һ�����Դ��ڴ�NH4Cl��Һ�����ԣ�����Һ����NH4Cl�������϶��á�
����֪��Һ�����Դ��ڴ�NH4Cl��Һ�����ԣ�����Һ����NH4Cl�������϶��á�
��3����NH3��H2O������������Ϻ���Һ�����ԣ�����c��H+��=c��OH-����c�� ��=c��Cl-��n��
��=c��Cl-��n�� ��=n��Cl-��������Һ����δ��Ӧ��NH3��H2O������ǰn��NH3��H2O����n��HCl��c��NH3��H2O����c��HCl����
��=n��Cl-��������Һ����δ��Ӧ��NH3��H2O������ǰn��NH3��H2O����n��HCl��c��NH3��H2O����c��HCl����
��1������Һֻ�ܽ�һ�����ʣ�ֻ��NH4Cl��Ϊ����ʱ����Һ�в�ͬʱ���������������ӡ���
 ��������ˮ�ⷴӦ
��������ˮ�ⷴӦ +H2O
+H2O NH3��H2O+H+��c��
NH3��H2O+H+��c�� ����С��c��H+����c��OH-��������Һ��c��Cl-����c��
����С��c��H+����c��OH-��������Һ��c��Cl-����c��
 ����c��H+����c��OH-����
����c��H+����c��OH-������2������Һ�д���c��
 ����c��Cl-����c��OH-����c��H+������c��OH-����c��H+����֪��Һ�ʼ��ԣ�����Һ����NH4Cl��NH3��H2O��϶��ã�������ΪNH4Cl��NH3��H2O������Һ�д���c��Cl-����c��H+����c��
����c��Cl-����c��OH-����c��H+������c��OH-����c��H+����֪��Һ�ʼ��ԣ�����Һ����NH4Cl��NH3��H2O��϶��ã�������ΪNH4Cl��NH3��H2O������Һ�д���c��Cl-����c��H+����c�� ����c��OH-������c��H+����c��
����c��OH-������c��H+����c�� ����֪��Һ�����Դ��ڴ�NH4Cl��Һ�����ԣ�����Һ����NH4Cl�������϶��á�
����֪��Һ�����Դ��ڴ�NH4Cl��Һ�����ԣ�����Һ����NH4Cl�������϶��á���3����NH3��H2O������������Ϻ���Һ�����ԣ�����c��H+��=c��OH-����c��
 ��=c��Cl-��n��
��=c��Cl-��n�� ��=n��Cl-��������Һ����δ��Ӧ��NH3��H2O������ǰn��NH3��H2O����n��HCl��c��NH3��H2O����c��HCl����
��=n��Cl-��������Һ����δ��Ӧ��NH3��H2O������ǰn��NH3��H2O����n��HCl��c��NH3��H2O����c��HCl����
��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
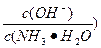 ��ֵ_________(�������С�����䡱)��
��ֵ_________(�������С�����䡱)��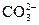 )֮�ȸ��ӽ�2��1�Ĵ�ʩ�ǣ���
)֮�ȸ��ӽ�2��1�Ĵ�ʩ�ǣ���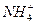 )�����ǣ���
)�����ǣ��� ����c��Cl-����c��H+����c��OH-��
����c��Cl-����c��H+����c��OH-��