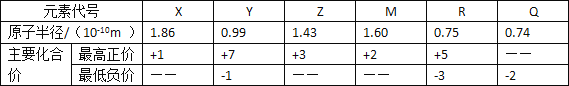
��Ŀ����
����Ŀ��ʵ����������ͼװ�ý����к��ȵIJⶨ���ش��������⣺
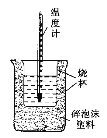
(1)��ͼ��������δ������������____________��____________��
(2)�ڲ�����ȷ��ǰ��������к��Ȳⶨ��ȷ�ԵĹؼ���____________��
(3)�����0.50 mol/L��������������ƹ������ʵ�飬��ʵ��������������к�������____________(����ƫ��������ƫС������������)��ԭ����____________��
(4)�Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϡ�2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43 kJ����������ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽΪ____________________________��
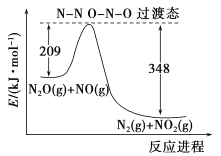
(5)��N2O��NO��Ӧ����N2��NO2�������仯��ͼ��ʾ��������1molN2������H=__________kJ��mol-1��
���𰸡����β�������� ֽ��� ���� ƫ�� �������ƹ����ܽ���� ![]() -139
-139
��������
(1)�������ȼƵĹ������жϸ�װ�õ�ȱ��������
(2)�������кͷ�Ӧ�У�����ȷ��������ɢʧ��
(3)�������ƹ�������ˮ�ų�������
(4)ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ų����������Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ������2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ������������1mol�Ҵ�ȼ����ȫȼ������Һ̬ˮ�ų����������ݴ���д�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ��
(5)��ͼ��֪������Ӧ�Ļ��Ϊ209kJ��mol-1���淴Ӧ�Ļ��Ϊ348kJ��mol-1������ͼ��֪���˷�ӦΪ���ȷ�Ӧ�������淴Ӧ�Ļ��ֻ����Ƿ�Ӧ���ʱ䡣
(1)���к��Ȳⶨʵ��Ĺ����֪����װ�õ�ȱ�������ǻ��β����������ֽ��ǣ�
(2)���кͷ�Ӧ�У�����к��Ȳⶨ��ȷ�ԵĹؼ���ȷ��������ɢʧ�������£�
(3)�������ƹ�������ˮ���ȣ��¶�ƫ�ߣ�����ʵ���в�õ��к�����ֵ��ƫ��
(4)�Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ������2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ����������1mol�Ҵ���ȫȼ������Һ̬ˮ�ų�������Ϊ��1366.89kJ������ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH(l)+3O2(g)�T2CO2(g)+3H2O(l)��H=1366.89kJmol1��
(5)��ͼ��֪���÷�ӦΪ���ȷ�Ӧ���˷�Ӧ�ķ�Ӧ��Ϊ���淴Ӧ�Ļ��֮����H=(209-348)kJ��mol-1=-139kJ��mol-1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ҵ�ϳɰ��ķ�Ӧ���£�3H2+N2![]() 2NH3��ij�¶��£����ݻ��㶨Ϊ2.0 L���ܱ������г���2.0 mol N2��2.0 mol H2��һ��ʱ���Ӧ��ƽ��״̬��ʵ���������±���ʾ��
2NH3��ij�¶��£����ݻ��㶨Ϊ2.0 L���ܱ������г���2.0 mol N2��2.0 mol H2��һ��ʱ���Ӧ��ƽ��״̬��ʵ���������±���ʾ��
t/s | 0 | 50 | 150 | 250 | 350 |
n(NH3)/mol | 0 | 0.24 | 0.36 | 0.40 | 0.40 |
(1)0��50 s�ڵ�ƽ����Ӧ���� v(N2)��_______________��
(2)250 sʱ��H2��ת����Ϊ_______________��
(3)��֪N��N�ļ���Ϊ946 kJ��mol-1��H��H�ļ���Ϊ436 kJ��mol-1��N��H�ļ���Ϊ391 kJ��mol-1��������1 mol NH3�����е������仯Ϊ_______kJ����ͼ����ȷ��ʾ�÷�Ӧ�������仯����_____������ĸ����
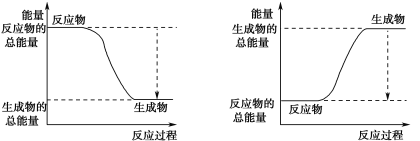
A B
(4)Ϊ�ӿ췴Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ______________��
a�������¶� b������ѹǿ c������ʱ����He��
d����ѹʱ����He�� e����ʱ�����NH3
(5)����˵���������____________��
a��ʹ�ô�����Ϊ�˼ӿ췴Ӧ���ʣ��������Ч��
b�����������£�N2������100%ת��ΪNH3
c����һ�������£��ϳɰ���Ӧ��һ������
d��250��350 sʱ��������Ũ�ȱ��ֲ��䣬��Ӧֹͣ