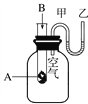
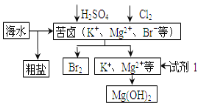
ћвƒњƒЏ»Ё
°Њћвƒњ°њѕ÷”–“‘ѕ¬ЉЄ÷÷”–їъќп£Ї
ҐўCH4 ҐЏCH3CH2OH Ґџ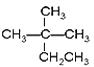 Ґ№єпЌйҐЁCH3COOHҐё
Ґ№єпЌйҐЁCH3COOHҐё![]() Ґя
Ґя![]() ҐаCH3CH2CH2CH3 Ґб±ыЌй
ҐаCH3CH2CH2CH3 Ґб±ыЌй
«лјы”√…ѕ цЄш≥цµƒќп÷ ∞і“™«уїЎірѕ¬Ѕ–ќ ћв£Ї
(1)ҐџµƒѕµЌ≥√ь√ы «_________£ї
(2)”륟ї•ќ™ЌђЈ÷“мєєћеµƒ «________£®ћо–тЇ≈£©£ї
(3)‘Џ120°ж£ђ1.01°Ѕ105PaћхЉюѕ¬£ђƒ≥÷÷∆шћђћю”л„гЅњµƒO2Ќк»ЂЈі”¶Їу£ђ≤вµ√Јі”¶«∞Їу∆шћеµƒћеїэ√ї”–ЈҐ…ъЄƒ±д£ђ‘тЄ√ћю «________£®ћо–тЇ≈£©£ї
(4)”√°∞£Њ°±±н ЊҐўҐџҐ№Ґб»џЈ–µгЄяµЌЋ≥–т£Ї__________£®ћо–тЇ≈£©£ї
(5)Ґаµƒґю¬»іъќпЌђЈ÷“мєєће эƒњ «_______÷÷£ї
(6)Њя”–ћЎ в∆шќґ£ђ≥£„чЁЌ»°ЉЅµƒ”–їъќп‘Џћъ„чіяїѓЉЅµƒћхЉюѕ¬”л“ЇдеЈҐ…ъ“ї»°іъЈі”¶µƒїѓ—ІЈљ≥ћ љ_________£ї
(7)ҐЏ‘ЏЉ”»»ћхЉюѕ¬ЇЌCuOЈі”¶µƒїѓ—ІЈљ≥ћ љ____________£ї
(8)““ґюіЉЇЌ„гЅњҐЁЈҐ…ъх•їѓЈі”¶µƒїѓ—ІЈљ≥ћ љ «_________°£
°Њір∞Є°њ2£ђ2°™ґюЉ„їщґ°Ќй Ґя Ґў Ґ№£ЊҐџ£ЊҐб£ЊҐў 6 ![]() +Br2
+Br2![]() +HBr CH3CH2OH+CuO
+HBr CH3CH2OH+CuO![]() CH3CHO+Cu+H2O 2CH3COOH + HO-CH2-CH2-OH
CH3CHO+Cu+H2O 2CH3COOH + HO-CH2-CH2-OH![]() CH3COOCH2-CH2-OOCCH3 + 2H2O
CH3COOCH2-CH2-OOCCH3 + 2H2O
°Њљвќц°њ
(1)Ґџµƒ÷чЅіЇђ”–4ЄцC‘≠„”£ђ2ЄцЉ„їщ„ч÷ІЅі£ђЋьµƒѕµЌ≥√ь√ы « 2£ђ2°™ґюЉ„їщґ°Ќй£ї
(2)ҐџЇЌҐяµƒїѓ—І љЊщќ™C6H14£ђ‘тЋь√«ї•ќ™ЌђЈ÷“мєєће£ђє ір∞Єќ™£ЇҐя£ї
(3)‘Џ120°ж£ђ1.01°Ѕ105PaћхЉюѕ¬£ђ…ъ≥…µƒЋЃќ™∆шћђ£ђ”…CxHy+£®x+![]() £©O2
£©O2![]() xCO2+
xCO2+![]() H2O£ђ‘т1+£®x+
H2O£ђ‘т1+£®x+![]() £© =x+
£© =x+![]() £ђњ…љвµ√y=4£ђ‘тCxHyќ™Љ„Ќй£ђє ір∞Єќ™£ЇҐў£ї
£ђњ…љвµ√y=4£ђ‘тCxHyќ™Љ„Ќй£ђє ір∞Єќ™£ЇҐў£ї
(4)ћЉ‘≠„”Єц э‘љґа£ђЈ–µг‘љіу£ђѕаЌђћЉ‘≠„”Єц эµƒЌйћю÷–÷ІЅіґаµƒЈ–µгµЌ£ђ‘тЈ–µгќ™Ґ№>Ґџ>Ґб>Ґў£ђє ір∞Єќ™£ЇҐ№>Ґџ>Ґб>Ґў£ї
(5)ҐаЉі’эґ°Ќй£ђЋь «“‘÷–Љдє≤ЉџЉьґ‘≥∆£ђ‘тЋьµƒґю¬»іъќпЌђЈ÷“мєєће эƒњ «6÷÷£ї
(6)Њя”–ћЎ в∆шќґ£ђ≥£„чЁЌ»°ЉЅµƒ”–їъќпќ™±љ£ђ‘Џћъ„чіяїѓЉЅµƒћхЉюѕ¬”л“ЇдеЈҐ…ъ“ї»°іъЈі”¶µƒїѓ—ІЈљ≥ћ љќ™![]() +Br2
+Br2![]()
![]() +HBr£ђір∞Єќ™£Ї
+HBr£ђір∞Єќ™£Ї![]() +Br2
+Br2![]()
![]() +HBr£ї
+HBr£ї
(7)ҐЏЉі““іЉ£ђ““іЉ‘ЏЉ”»»ћхЉюѕ¬ЇЌCuOЈі”¶…ъ≥…““»©ЇЌЋЃ£ђїѓ—ІЈљ≥ћ љќ™£ЇCH3CH2OH+CuO![]() CH3CHO+Cu+H2O£ђє ір∞Єќ™£ЇCH3CH2OH+CuO
CH3CHO+Cu+H2O£ђє ір∞Єќ™£ЇCH3CH2OH+CuO![]() CH3CHO+Cu+H2O£ї
CH3CHO+Cu+H2O£ї
(8)““ґюіЉЇЌ„гЅњҐЁЉі““ЋбЈҐ…ъх•їѓЈі”¶…ъ≥…““Ћб““ґюх•£ђїѓ—ІЈљ≥ћ љ «ќ™£Ї2CH3COOH + HO-CH2-CH2-OH![]() CH3COOCH2-CH2-OOCCH3 + 2H2O°£
CH3COOCH2-CH2-OOCCH3 + 2H2O°£

 —ІЅЈњм≥µµјњЏЋг–ƒЋгЋўЋгћмћмЅЈѕµЅ–ір∞Є
—ІЅЈњм≥µµјњЏЋг–ƒЋгЋўЋгћмћмЅЈѕµЅ–ір∞Є