��Ŀ����
����Ŀ��GaN������5GоƬ�IJ��ϣ����������͵�����LED�ɷ�������⡣�ش��������⣺
(1)��̬Asԭ�Ӻ�������Ų�ʽΪ[Ar]________������״̬�����У�����������һ����������������С����_____________(����)��
A. ![]() B.
B. ![]() C.[Ne] D.
C.[Ne] D. ![]()
(2)8���ǻ������(����ʽC27H18AlN3O3)���ڷ�����ϼ����Ӵ�����ϣ�����LiAlH4�� (8���ǻ����)�ϳɡ�LiAlH4�������ӵĿռ乹��Ϊ_______��
(8���ǻ����)�ϳɡ�LiAlH4�������ӵĿռ乹��Ϊ_______�� ����Ԫ���е縺��������___(��Ԫ�ط���)��C��N��O���ӻ���ʽ����Ϊ _____��_________��____________��
����Ԫ���е縺��������___(��Ԫ�ط���)��C��N��O���ӻ���ʽ����Ϊ _____��_________��____________��
(3)��֪���л�������۵㣺
������ | AlF3 | GaF3 | AlCl3 |
�۵�/�� | 1040 | 1000 | 194 |
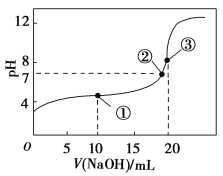
�ٱ���±������۵���������ԭ����____________��
������AlCl3ʱ�����ɾ��лӷ��ԵĶ�����Al2Cl6��������Al2Cl6�ĽṹʽΪ_______������Al����λ��Ϊ_________��
(4)GaAs�ľ����ṹ��ͼ��ʾ�����ڵ�Asԭ��֮��ľ���Ϊx,���ڵ�As��Gaԭ��֮��ľ���Ϊy����![]() =________��
=________��

���𰸡�3d104s24p3 D �������� O sp2 sp2 sp3 AlF3��GaF3Ϊ���Ӿ��壬AlCl3Ϊ���Ӿ��壬�����ܣ�AlF3>GaF3 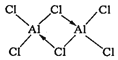 4
4 ![]()
��������
(1)AsΪ33��Ԫ�أ���ԭ�Ӻ�����33�����ӣ����ݹ���ԭ����д��̬ԭ�Ӻ�������Ų�ʽ������������һ������������������̬������̬����һ�����ܣ��ڶ������ܣ����������ܣ�
(2)LiAlH4��������ΪAlH4-�����ݼ۲���ӶԸ���=��������+�µ��ӶԸ��������жϸ���������Alԭ�Ӽ۲���ӶԸ��������жϸ������ӿռ乹�ͣ�Ԫ�صķǽ�����Խǿ����縺��Խ��8һ�ǻ�����л��ϵ�C��N��Oԭ�Ӽ۲���ӶԸ����ֱ�Ϊ3��3��4�����ݼ۲���ӶԻ��������ж�ԭ���ӻ����ͣ�
(3)��ԭ�Ӿ����۷е�ϸߡ����Ӿ����۷е�ϵͣ��ڶ�����Al2Cl6��Alԭ���пչ����Cl�����ṩ�µ��Ӷ��γ���λ����������е���λ����ֱָ��ͬ��������(��ԭ��)��λ��ԭ����Ŀ��
(4)���ݾ����ṹͼ������������ڵ�����Asԭ�ӵľ���Ϊ���㵽���ĵľ���ͽ��ڵ�As��Gaԭ��֮��ľ��롣
(1)AsΪ33��Ԫ�أ���̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s24p3�����ĵ��������ܣ��ڶ������ܣ���һ�����ܣ���̬���ڼ���̬������D�����������ͣ��ʴ�Ϊ��3d104s24p3��D��
(2)LiAlH4��������ΪAlH4-������������Alԭ�Ӽ۲���ӶԸ���=4+![]() =4�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ������ӿռ乹��Ϊ�������壻Ԫ�صķǽ�����Խǿ����縺��Խ�ǽ�����O��N��C��H��Al����縺��O��N��C��H��Al��8һ�ǻ�����л��ϵ�C��N��Oԭ�Ӽ۲���ӶԸ����ֱ�Ϊ3��3��4�����ݼ۲���ӶԻ��������ж�ԭ���ӻ����ͣ�C��N��O�ӻ���ʽ�ֱ�Ϊ sp2��sp2��sp3���ʴ�Ϊ���������壻O��sp2��sp2��sp3��
=4�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ������ӿռ乹��Ϊ�������壻Ԫ�صķǽ�����Խǿ����縺��Խ�ǽ�����O��N��C��H��Al����縺��O��N��C��H��Al��8һ�ǻ�����л��ϵ�C��N��Oԭ�Ӽ۲���ӶԸ����ֱ�Ϊ3��3��4�����ݼ۲���ӶԻ��������ж�ԭ���ӻ����ͣ�C��N��O�ӻ���ʽ�ֱ�Ϊ sp2��sp2��sp3���ʴ�Ϊ���������壻O��sp2��sp2��sp3��
(3)�����Ӿ�����۷е�ϸߡ����Ӿ����۷е�ϵͣ�����������ɼ��۵��ȷ��AlF3��GaF3Ϊ���Ӿ��壬AlCl3Ϊ���Ӿ��壬���Ӿ����۵�һ��ȷ��Ӿ���ĸߣ����Ӿ����۵��ɾ�������Դ�С�����������ܣ�AlF3��GaF3�������۷е㣺AlF3��GaF3��AlCl3���ʴ�Ϊ��AlF3��GaF3Ϊ���Ӿ��壬AlCl3Ϊ���Ӿ��壬�����ܣ�AlF3��GaF3��
������ʱAlCl3���ɿɻӷ��Ķ�����Al2Cl6���ö�������Al�ṩ�չ����Clԭ���ṩ���Ӷ��γ���λ������ṹʽΪ ��������е���λ����ֱָ��ͬ��������(��ԭ��)��λ��ԭ����Ŀ��������Al2Cl6����Alֱ��������ԭ����4����������λ��Ϊ4���ʴ�Ϊ��
��������е���λ����ֱָ��ͬ��������(��ԭ��)��λ��ԭ����Ŀ��������Al2Cl6����Alֱ��������ԭ����4����������λ��Ϊ4���ʴ�Ϊ�� ��4��
��4��
(4)���ڵ�����Asԭ�ӵľ���Ϊ���㵽���ĵľ���x=![]() apm�����ڵ�As��Gaԭ��֮��ľ���Ϊy=
apm�����ڵ�As��Gaԭ��֮��ľ���Ϊy=![]() ��
��![]() apm����x��y=
apm����x��y=![]() apm��(
apm��(![]() ��
��![]() apm)=
apm)=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�