��Ŀ����
����Ŀ��Ϊ�о��Ҵ��ṹ���䲿�ֵĻ�ѧ���ʣ���������ʵ�顣����������⣺
������ͼװ�����Ʋ��Ҵ��Ľṹʽ��
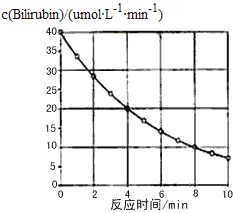
(1)ʵ��������Ҫ����ɺ�С�Ŀ�������ԭ���� _______________________________��
(2)����ʵ��ƽ�����Ҵ�1.15g���ռ���H2���ƽ��Ϊ0.28L(����ɱ�״̬)����ʵ�����ݿ��Ʋ�H2�����Ҵ�������________(������)����ԭ�ӡ�
(3)ʢװ�Ҵ��IJ���������________________________
����������װ�ý����Ҵ��Ĵ�����ʵ�飬��������������Cװ�õ��Թ���ʢ����ˮ�Ҵ���(�̶��ͼг�װ������ȥ)
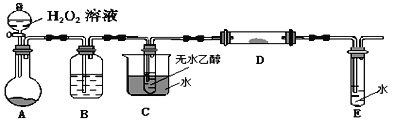
(4)װ��AԲ����ƿ�ڵĹ���������_____��B�е��Լ���_____C��������______��
(5)��ʵ������Ҫ���ȵ�װ����_________________ (��װ���µ���ĸ)��
(6)д��D��������Ӧ�Ļ�ѧ����ʽ_________________________________��
(7)����E�е���������Ҫ���Լ�Ϊ______________________��
���𰸡�����Ӵ�������߷�Ӧ���� �ǻ� ��Һ©�� MnO2 Ũ���� Ԥ�ȷ�Ӧ������� (�ṩ�Ҵ�����) CD 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ������Һ(������������ͭ����Һ)
2CH3CHO+2H2O ������Һ(������������ͭ����Һ)
��������
��(1)�Ʒ���������Ӵ��棬��߷�Ӧ���ʣ�
(2)������Ҵ������ʵ����������ƹ������Ҵ���ȫ��Ӧ���������������ʵ���������Ҵ����ܹ�����ȡ����ԭ������������ԭ����ȷ���Ҵ��Ľṹ��
(3)���������ṹȷ���Ҵ�ʢ�ŵ��������ƣ�
��A���ǹ��������ڶ������̴�����������ˮ��������B�����������е�ˮ������C�Ǽ����Ҵ��õ��Ҵ���������D��D�м���ʱ�Ҵ�������Ϊ��ȩ����E�����շ�Ӧ��������ȩ������ʵ��Ŀ��ȷ�����������ü�ʹ�÷�����
��(1)ʵ��������Ҫ����ɺ�С�Ŀ�����Ŀ������Ӵ��������߷�Ӧ���ʣ�
(2)�Ҵ�������Ϊ��m(C2H6O)=1.15g�����Ҵ������ʵ���Ϊ��n(C2H6O)=1.15g ��46g/mol=0.025mol���ռ����������ƽ��Ϊ0.28��(����ɱ�״̬)�������ʵ���Ϊn(H2)= 0.28L��22.4L/mol=0.0125mol Ҳ����Ϊ0.025molH���ɼ�1��C2H6O�����У�ֻ��1��H���Ա�Na�û�����˵��C2H6O�������6��H�У���1��������5���Dz�ͬ�ģ��Ҵ��Ľṹ��ʽΪCH3CH2OH�����Ʋ�H2�����Ҵ��������ǻ�Hԭ�ӣ�
(3)����װ��ͼ��֪ʢװ�Ҵ��IJ��������Ƿ�Һ©����
��(4)A����H2O2��MnO2������������ˮ����������Ӧ�Ļ�ѧ����ʽΪ2H2O2 ![]() 2H2O+O2����B�������������������е�ˮ����������Ũ��������ˮ����C�������Ǽ����Ҵ��õ��Ҵ���������D������C������ΪԤ�ȷ�Ӧ������壻
2H2O+O2����B�������������������е�ˮ����������Ũ��������ˮ����C�������Ǽ����Ҵ��õ��Ҵ���������D������C������ΪԤ�ȷ�Ӧ������壻
(5)�ڱ�ʵ���У�C����Ȳ����Ҵ�������E����ȣ�ʹ�Ҵ�������O2��Cu���·���������Ӧ������ȩ������Ҫ���ȵ�װ����CD��
(6)��Dװ�����Ҵ�������������ȩ����Ӧ�ķ���ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(7)�Ҵ�������Ϊ��ȩ����ȩ����ȩ�������л�ԭ�ԣ��ܱ�������Һ������������ͭ����Һ�����������֤�Ҵ�����������ȩ�Ļ�ѧ�Լ�Ϊ������Һ������������ͭ����Һ��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д�����Ŀ���̷�(FeSO4��7H2O)�ڹ�ҵ�Ͽ������������Ρ��������켰����ȡ���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����

25�� | pHֵ |
����H2S��Һ | 3.9 |
SnS������ȫ | 1.6 |
FeS��ʼ���� | 3.0 |
FeS��ʼ���� | 5.5 |
��1�������Ƶõ��̷��������Ƿ���Fe3�������ѡ�õ��Լ�Ϊ________��
A��KSCN��Һ B��NaOH��Һ C��KMnO4��Һ D��������Һ
��2���������У�ͨ�����������͵�Ŀ����______________________________________������Һ���������ữ��pH��2��Ŀ����__________________________________��
��3����������˳������Ϊ_________________����ȴ�ᾧ��____________________��
��4���������õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ���_________________________________________________________��
��5���ⶨ�̷���Ʒ��Fe2�������ķ����ǣ�a.��ȡ2.850 g�̷���Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.�������ữ��0.010 00 mol��L��1 KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00 mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ________________________(����������)��
�ڼ���������Ʒ��FeSO4��7H2O����������Ϊ____________________��