��Ŀ����
����Ŀ����Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�ĺ��������ǵ���������ٵ��Ͼ�����,�ش���������:
��1���ҹ���������������úΪ��Ҫȼ�ϵĹ���,���й���ú��ȼ�ϵ��۵���ȷ���� ___________________(����ĸ)��
A��ú����Ҫ�Ļ���ԭ�ϣ���ú��ȼ�ϼ�ȼ�յ�̫��ϧ,Ӧ���ۺ�����
B��ú�Ƿ������ܸߵĹ���ȼ�ϣ��ҹ�ú̿��Դ��Լ��У����ɳɱ��ͣ���ú��ȼ��ʵ��
C��úȼ��ʱ������������������̳����Ի�����Ⱦ����
D��ͨ���ྻú��������ú��������Һ�����Լ�����������������ȼú��Ⱦ���������úȼ�յ���������
��2���Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϡ�2.0 g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43 kJ�����������Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ____________________________________________________________��
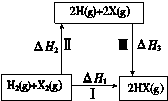
��3������C3H8(g)��C3H6(g)+H2(g) ��H=+b kJmol1(b��0)�ķ�Ӧ�У���Ӧ����е�������________(����������������������С����)��������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ________(�����ų�������������)��������ת��Ϊ�����
��4��������ˮ��ȡ������Դ�����������о�������ȷ����________________
A�����ˮ����������ǿ���ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B���跨��̫����۽����������£�ʹˮ�ֽ��������
C��Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ͬʱ�ͷ�����
D��Ѱ��������������ڿ���������Դ���Էֽ�ˮ��ȡ����
��5����֪���������Ȼ�ѧ����ʽ��
A��2H2(g)+O2(g) ===2H2O(l) ��H��-571.6 kJmol-1 B��C3H8(g)+5O2(g) ===3CO2(g)+4 H2O(l) ��H��-2 220 kJmol-1�����У��ܱ�ʾȼ���ȵ��Ȼ�ѧ����ʽΪ___________��A��B������ȼ����Ϊ______________��
���𰸡�ACDC2H5OH(l)+3O2(g)![]() 2CO2(g)+3H2O(l)����H=��1 366.89 kJ��mol-1С������ACB2 220 kJmol-1
2CO2(g)+3H2O(l)����H=��1 366.89 kJ��mol-1������ACB2 220 kJmol-1
��������
���⿼���˹���ɵ�Ӧ���Լ���Ӧ�������뷴Ӧ�����������֮��Ĺ�ϵ��
��1��ú��ȼ�գ�ȼ��ʱ������������������̳�����ȼ��Ч�ʽϵͣ���ͨ���ྻú��������ú��������Һ���Լ������������úȼ�յ��������ʣ�����úֱ����ȼ�ϣ��ɵ��»�����Ⱦ����ɽϴ��˷ѣ�ACD��ȷ����2���Ҵ���ȫȼ�����ɶ�����̼��Һ̬ˮ������2.0g�Ҵ���ȫȼ������Һ̬ˮ�ų�59.43kJ����������1mol�Ҵ���ȫȼ������Һ̬ˮ�ų�������Ϊ��1366.89KJ������ȼ���ȵ��Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l����H=-1366.89kJmol-1����3������C3H8��g���TC3H6��g��+H2��g���ķ�Ӧ�С�H��0���÷�Ӧ�����ȷ�Ӧ����Ӧ����е�������С�������������������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ��Ҫ����������������ת��Ϊ�������4����������ȼ�գ�������ȼ�գ�ˮ�е���Ԫ��Ҳ����ȼ�գ�A�����跨��̫���ܾ۽����������£�ʹˮ�ֽ����H2��B��ȷ��Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ˮ�ֽ���Ҫ����������C����Ѱ�����⻯ѧ���������������ڿ���������Դ���Էֽ�ˮ��ȡH2��D��ȷ����ѡAC����5��ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Ը����Ȼ�ѧ����ʽ��֪��������ȼ������571.6 kJmol-1��2��285.8 kJmol-1��1mol������ȫȼ������4molҺ̬ˮʱ������2 220 kJ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ú��һ����Ҫ�Ļ���ԭ�ϣ����ǽ�����ú��ȡ��ˮú������̿�����ѵȹ㷺���ڹ�ũҵ�����С�
��1����֪����C(s)+H2O(g)�TCO(g)+H2(g) ��H=+131.3kJ��mol-1
��CO2(g)+H2(g)�TCO(g)+H2O(g) ��H=+41.3kJ��mol-1
��̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽΪ���÷�Ӧ��(����¡��������¡����κ��¶ȡ�)�������������Է����С�
��2����������̿��ԭ�������������������ӦC(s)+2NO(g) ![]() N2(g)+CO2(g)����ij�ܱ������м���һ�����Ļ���̿��NO����T1��ʱ����ͬʱ���ø����ʵ�Ũ�����±���ʾ��
N2(g)+CO2(g)����ij�ܱ������м���һ�����Ļ���̿��NO����T1��ʱ����ͬʱ���ø����ʵ�Ũ�����±���ʾ��
ʱ��(min) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
��10��20min�ڣ�N2��ƽ����Ӧ����v(N2)=��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı����������������ĸ���)��
A��ͨ��һ������NO B������һ�����Ļ���̿
C��������ʵĴ��� D���ʵ���С���������
��3���о���������ӦCO(g)+H2O(g) ![]() H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2(g)+CO2(g)ƽ�ⳣ�����¶ȵı仯���±���ʾ��
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
����Ӧ��500��ʱ���У�����ʼʱCO��H2O��Ũ�Ⱦ�Ϊ0.020mol��L-1 �� �ڸ������´ﵽƽ��ʱ��CO��ת����Ϊ��