��Ŀ����
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
���� ����ʽ��HCl ��Է���������36.5,�ܶȣ�1.19 g��cm��3 HCl������������36.5% |
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__ ____mol��L��1��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����________��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ���ᡣ
����ѧ����Ҫ��ȡ___ _____mL����Ũ�������������
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(����������A��ʾ��ƫ��������B��ʾ��ƫС������C��ʾ����Ӱ����)��
a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�� ( )
b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ ( )
��4���������ͬѧ�ɹ�������0.400 mol��L��1�����ᣬ�����ø������кͺ�0.4 g NaOH��NaOH��Һ�����ͬѧ��ȡ________mL���ᡣ����ȷ��С�����һλ��
�������ͬѧ�������Ƶ������кͺ�0.4 g NaOH��NaOH��Һ�����ֱ������������ƫС������ܵ�ԭ����________��
A��Ũ����ӷ���Ũ�Ȳ���
B��������Һʱ��δϴ���ձ�
C��������Һʱ����������ƿ�̶���
D����ˮʱ�����̶��ߣ��ý�ͷ�ι�����
(1)11.9 (2)BD (3)��16.8 ��a.B b��B (4)��25 ��C
��������
�����������1������c��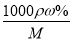 ��֪����Ũ������HCl�����ʵ���Ũ��c��
��֪����Ũ������HCl�����ʵ���Ũ��c��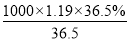 mol/L��11.9mol/L��
mol/L��11.9mol/L��
��2����Һ�Ǿ�һ���ȶ��Ļ�������ȡ����������ĸ�������Һʱ��A������n��cV��֪����Һ��HCl�����ʵ�������Һ������й�ϵ��A����ȷ��B����Һ��Ũ���Dz���ģ�����11.9mol/L��B��ȷ��C����Һ��Cl������Ŀ���Ȼ�������ʵ����й�ϵ���������Һ������й�ϵ��C����ȷ��D����Һ���ܶ�����Һ�����ʣ�����Һ�������ϵ��D��ȷ����ѡBD��
��3���ٸ���ϡ���������ʵ����������֪������500 mL���ʵ���Ũ��Ϊ0.400 mol��L��1��ϡ������Ҫ��Ũ����������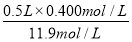 ��0.0168L��16.8ml��
��0.0168L��16.8ml��
������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�������������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��a������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ������Ũ�����ƫС�����������Һ��Ũ��ƫ�ͣ���ѡB��b�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ������Һ��������ӣ�����������Һ��Ũ��ƫ�ͣ���ѡB��
��4����0.4 g NaOH�����ʵ�����0.4g��40g/mol��0.01mol������ݷ���ʽHCl��NaOH=NaCl��H2O��֪�������Ȼ�������ʵ�����0.01mol�������Ҫ������������0.01mol��0.400mol/L��0.025L��25ml��
��A��Ũ����ӷ���Ũ�Ȳ����������������Ƶ����ʵ�������������£�����������Һ�����ƫ��A����ȷ��B��������Һʱ��δϴ���ձ������������Ũ��ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫ��B����ȷ��C��������Һʱ����������ƿ�̶�����������ƿ����Һ�����ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫС��C��ȷ��D����ˮʱ�����̶��ߣ��ý�ͷ�ι����������������Ũ��ƫС��������������Ƶ����ʵ�������������£�����������Һ�����ƫ��D����ȷ����ѡC��
���㣺�������ʵ���Ũ�ȵ��йؼ��㡢���ơ��������Ե�

 �߽�������ϵ�д�
�߽�������ϵ�д�