ΧβΡΩΡΎ»ί
Β―ιΧΫΨΩΘΚΧΫΨΩΧΦΓΔΙη‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΒΡœύΕ‘«Ω»θ
ΗυΨί“Σ«σΆξ≥…œ¬Ν–Ης–ΓΧβ
Θ®1Θ© Β―ιΉΑ÷ΟΘΚ
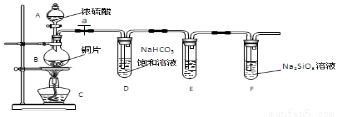
Χν–¥Υυ Ψ“«ΤςΟϊ≥ΤA B
Θ®2Θ© Β―ι≤Ϋ÷ηΘΚ
Ν§Ϋ”“«ΤςΓΔ ΓΔΦ”“©ΤΖΚσΘ§¥ρΩΣaΓΔ»ΜΚσΒΈ»κ≈®ΝρΥαΘ§Φ”»»
Θ®3Θ©Έ ΧβΧΫΨΩΘΚΘ®“―÷ΣΥα–‘«Ω»θ:―«ΝρΥα >ΧΦΥαΘ©
ΔΌΆ≠”κ≈®ΝρΥαΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ « ΘΜΉΑ÷ΟE÷–ΉψΝΩΥα–‘KMnO4»ή“ΚΒΡΉς”Ο « ΘΜ
ΔΎΡήΥΒΟςΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘±»Ιη‘ΣΥΊΖ«Ϋπ τ–‘«ΩΒΡ Β―ιœ÷œσ « ΘΜ
Δέ“άΨί ‘ΙήD÷–ΒΡ Β―ιœ÷œσΘ§ΡήΖώ÷ΛΟςΝρ‘ΣΥΊΒΡΖ«Ϋπ τ–‘«Ω”ΎΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘ Θ®ΧνΓΑΡήΓ±ΜρΓΑΖώΓ±Θ©Θ§ ‘ΙήD÷–ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «
Θ®1Θ©AΘΚΖ÷“Κ¬©ΕΖBΘΚ‘≤ΒΉ…’ΤΩ
Θ®2Θ©Φλ≤ιΉΑ÷ΟΒΡΤχΟή–‘
Θ®3Θ©ΔΌCu+2H2SO4(≈®) CuSO4+ SO2Γϋ+2H2O ≥ΐ»ΞSO2ΤχΧε
CuSO4+ SO2Γϋ+2H2O ≥ΐ»ΞSO2ΤχΧε
ΔΎ Δ”–Na2SiO3»ή“ΚΒΡ ‘Ιή÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμ
Δέ Ζώ SO2+2HCO3-=SO32-+ H2O+CO2ΓϋΜρSO2+HCO3-=HSO3-+CO2Γϋ
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΘ®1Θ©A : Ζ÷“Κ¬©ΕΖBΘΚ‘≤ΒΉ…’ΤΩ Θ°Θ®2Θ© Ε‘”Ύ”–ΤχΧε≤ΈΦ”ΒΡΖ¥”ΠΜρ÷Τ»ΓΤχΧεΒΡΖ¥”ΠΘ§‘ΎΉΑ“©ΤΖ÷°«Α“ΜΕ®“Σ Ήœ»Φλ≤ιΉΑ÷ΟΒΡΤχΟή–‘ΓΘ»ΜΚσ≤≈Ω…“‘Ϋχ–– Β―ιΓΘΘ®3Θ©ΔΌCu”κ≈®H2SO4‘ΎΦ”»» ±ΖΔ…ζΖ¥”ΠΓΘΖ¥”ΠΒΡΖΫ≥Χ ΫΈΣΘΚCu+2H2SO4(≈®)  CuSO4+ SO2Γϋ+2H2O ΓΘΉΑ÷ΟD Δ”–NaHCO3±ΞΚΆ»ή“ΚΘ§“ρΈΣΥα–‘H2SO3> H2CO3Υυ“‘Εΰ’ΏΖΔ…ζΖ¥”Π≤ζ…ζCO2Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣNaHCO3+ SO2= NaHSO3+ CO2Θ§ΉΑ÷ΟE÷– Δ”–Υα–‘KMnO4»ή“ΚΘ§ΡήΑ―SO2―θΜ·ΈΣΝρΥαΘ§ΒΪ «”κCO2≤ΜΖΔ…ζΖ¥”ΠΓΘΥυ“‘ΉΑ÷ΟE÷–ΉψΝΩΥα–‘KMnO4»ή“ΚΒΡΉς”Ο «≥ΐ»ΞΈΣΆξ»ΪΖ¥”ΠSO2ΤχΧεΓΘΔΎ»γΙϊΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘±»Ιη‘ΣΥΊΖ«Ϋπ τ–‘«ΩΘ§‘ρΥα–‘H2CO3> H2SiO3Α―CO2Ά®»κΒΫNa2SiO3ΒΡ»ή“Κ÷–ΨΆΜαΖΔ…ζΖ¥”ΠΘΚCO2+H2O +Na2SiO3= Na2CO3+ H2SiO3ΓΐΓΘΩ¥ΒΫ Β―ιœ÷œσ « Δ”–Na2SiO3»ή“ΚΒΡ ‘Ιή÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμΓΘΔέ“ρΈΣ‘ΣΥΊΒΡΖ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΒΡΥα–‘ΨΆ‘Ϋ«ΩΓΘΒΪ «H2SO3≤Μ «SΒΡΉνΗΏΦέΚ§―θΥαΓΘ“ρ¥Υ≤ΜΡή“άΨί ‘ΙήD÷–ΒΡ Β―ιœ÷œσΘ§÷ΛΟςΝρ‘ΣΥΊΒΡΖ«Ϋπ τ–‘«Ω”ΎΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΓΘ ‘ΙήD÷–»τΆ®»κΒΡSO2…ΌΝΩΘ§ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «SO2+2HCO3-=SO32-+ H2O+CO2ΓϋΘΜΜρ»τΆ®»κΒΡSO2ΉψΝΩΘ§ΖΔ…ζΖ¥”ΠΒΡΝΫ÷÷ΖΫ≥Χ Ϋ «SO2+HCO3-= HSO3- +CO2ΓϋΓΘ
CuSO4+ SO2Γϋ+2H2O ΓΘΉΑ÷ΟD Δ”–NaHCO3±ΞΚΆ»ή“ΚΘ§“ρΈΣΥα–‘H2SO3> H2CO3Υυ“‘Εΰ’ΏΖΔ…ζΖ¥”Π≤ζ…ζCO2Θ§Ζ¥”ΠΒΡΖΫ≥Χ ΫΈΣNaHCO3+ SO2= NaHSO3+ CO2Θ§ΉΑ÷ΟE÷– Δ”–Υα–‘KMnO4»ή“ΚΘ§ΡήΑ―SO2―θΜ·ΈΣΝρΥαΘ§ΒΪ «”κCO2≤ΜΖΔ…ζΖ¥”ΠΓΘΥυ“‘ΉΑ÷ΟE÷–ΉψΝΩΥα–‘KMnO4»ή“ΚΒΡΉς”Ο «≥ΐ»ΞΈΣΆξ»ΪΖ¥”ΠSO2ΤχΧεΓΘΔΎ»γΙϊΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘±»Ιη‘ΣΥΊΖ«Ϋπ τ–‘«ΩΘ§‘ρΥα–‘H2CO3> H2SiO3Α―CO2Ά®»κΒΫNa2SiO3ΒΡ»ή“Κ÷–ΨΆΜαΖΔ…ζΖ¥”ΠΘΚCO2+H2O +Na2SiO3= Na2CO3+ H2SiO3ΓΐΓΘΩ¥ΒΫ Β―ιœ÷œσ « Δ”–Na2SiO3»ή“ΚΒΡ ‘Ιή÷–≥ωœ÷ΑΉ…Ϊ≥ΝΒμΓΘΔέ“ρΈΣ‘ΣΥΊΒΡΖ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΒΡΥα–‘ΨΆ‘Ϋ«ΩΓΘΒΪ «H2SO3≤Μ «SΒΡΉνΗΏΦέΚ§―θΥαΓΘ“ρ¥Υ≤ΜΡή“άΨί ‘ΙήD÷–ΒΡ Β―ιœ÷œσΘ§÷ΛΟςΝρ‘ΣΥΊΒΡΖ«Ϋπ τ–‘«Ω”ΎΧΦ‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΓΘ ‘ΙήD÷–»τΆ®»κΒΡSO2…ΌΝΩΘ§ΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «SO2+2HCO3-=SO32-+ H2O+CO2ΓϋΘΜΜρ»τΆ®»κΒΡSO2ΉψΝΩΘ§ΖΔ…ζΖ¥”ΠΒΡΝΫ÷÷ΖΫ≥Χ Ϋ «SO2+HCO3-= HSO3- +CO2ΓϋΓΘ
ΩΦΒψΘΚΩΦ≤ι“«ΤςΒΡ Ε±πΓΔΜ·―ß Β―ιΜυ±Ψ≤ΌΉςΓΔΜ·―ßΖΫ≥Χ ΫΒΡ ι–¥ΓΔΈο÷ –‘÷ ΒΡΦλ―ιΓΔ‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΒΡ≈–ΕœΒΡ÷Σ ΕΓΘ

»γΆΦΈΣ Β―ι “Ρ≥≈®―ΈΥα ‘ΦΝΤΩ±ξ«©…œΒΡ”–ΙΊ ΐΨίΘ§ ‘ΗυΨί±ξ«©…œΒΡ”–ΙΊ ΐΨίΜΊ¥πœ¬Ν–Έ ΧβΘΚ
―ΈΥα Ζ÷Ή” ΫΘΚHCl œύΕ‘Ζ÷Ή”÷ ΝΩΘΚ36.5,ΟήΕ»ΘΚ1.19 gΓΛcmΘ≠3 HClΒΡ÷ ΝΩΖ÷ ΐΘΚ36.5% |
Θ®1Θ©ΗΟ≈®―ΈΥα÷–HClΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ__ ____molΓΛLΘ≠1ΓΘ
Θ®2Θ©»Γ”Ο»Έ“βΧεΜΐΒΡΗΟ―ΈΥα»ή“Κ ±Θ§œ¬Ν–ΈοάμΝΩ÷–≤ΜΥφΥυ»ΓΧεΜΐΒΡΕύ…ΌΕχ±δΜ·ΒΡ «________ΓΘ
AΘ°»ή“Κ÷–HClΒΡΈο÷ ΒΡΝΩ BΘ°»ή“ΚΒΡ≈®Ε»
CΘ°»ή“Κ÷–ClΘ≠ΒΡ ΐΡΩ DΘ°»ή“ΚΒΡΟήΕ»
Θ®3Θ©Ρ≥―ß…ζ”ϊ”Ο…œ ω≈®―ΈΥαΚΆ’τΝσΥ°≈δ÷Τ500 mLΈο÷ ΒΡΝΩ≈®Ε»ΈΣ0.400 molΓΛLΘ≠1ΒΡœΓ―ΈΥαΓΘ
ΔΌΗΟ―ß…ζ–η“ΣΝΩ»Γ___ _____mL…œ ω≈®―ΈΥαΫχ––≈δ÷ΤΓΘ
ΔΎ‘Ύ≈δ÷ΤΙΐ≥Χ÷–Θ§œ¬Ν– Β―ι≤ΌΉςΕ‘Υυ≈δ÷ΤΒΡœΓ―ΈΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»”–ΚΈ”ΑœλΘΩ(‘Ύά®Κ≈ΡΎΧνA±μ ΨΓΑΤΪ¥σΓ±ΓΔΧνB±μ ΨΓΑΤΪ–ΓΓ±ΓΔΧνC±μ ΨΓΑΈό”ΑœλΓ±)ΓΘ
aΘ°”ΟΝΩΆ≤ΝΩ»Γ≈®―ΈΥα ±Η© ”Ιέ≤λΑΦ“ΚΟφ ( )
bΘ°Ε®»ίΚσΨ≠’ώΒ¥ΓΔ“Γ‘»ΓΔΨ≤÷ΟΘ§ΖΔœ÷“ΚΟφœ¬ΫΒΘ§‘ΌΦ” ΝΩΒΡ’τΝσΥ° ( )
Θ®4Θ©ΔΌΦΌ…ηΗΟΆ§―ß≥…ΙΠ≈δ÷ΤΝΥ0.400 molΓΛLΘ≠1ΒΡ―ΈΥαΘ§Υϊ”÷”ΟΗΟ―ΈΥα÷–ΚΆΚ§0.4 g NaOHΒΡNaOH»ή“ΚΘ§‘ρΗΟΆ§―ß–η»Γ________mL―ΈΥαΓΘΘ®ΨΪ»ΖΒΫ–Γ ΐΒψΚσ“ΜΈΜΘ©
ΔΎΦΌ…ηΗΟΆ§―ß”Ο–¬≈δ÷ΤΒΡ―ΈΥα÷–ΚΆΚ§0.4 g NaOHΒΡNaOH»ή“ΚΘ§ΖΔœ÷±»ΔΌ÷–Υυ«σΧεΜΐΤΪ–ΓΘ§‘ρΩ…ΡήΒΡ‘≠“ρ «________ΓΘ
AΘ°≈®―ΈΥαΜ”ΖΔΘ§≈®Ε»≤ΜΉψ
BΘ°≈δ÷Τ»ή“Κ ±Θ§Έ¥œ¥Β”…’±≠
CΘ°≈δ÷Τ»ή“Κ ±Θ§Η© ”»ίΝΩΤΩΩΧΕ»œΏ
DΘ°Φ”Υ° ±≥§ΙΐΩΧΕ»œΏΘ§”ΟΫΚΆΖΒΈΙήΈϋ≥ω