��Ŀ����
����Ŀ������װ������ȼ�շ�ȷ���л������ʽ���õ�װ�á�
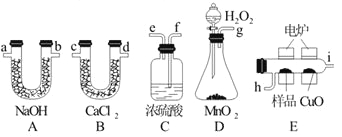
��֪������O2�������ҵ�������ѡװ�ø����ܵ���ȷ����˳����g��f��e��h��i��c��d��a��b��f��
��1��Cװ����Ũ�����������________________________��
��2��Dװ����MnO2��������_______________________��
��3��ȼ�չ���CuO��������_____________________��
��4����ʵ������ȡ��Ʒֻ��C��H��O����Ԫ���е����ֻ����֣�ȷ��ȡ0.92 g��Ʒ������ַ�Ӧ��A�� ��������1.76 g��B����������1.08 g�������Ʒ�Ļ�ѧʽΪ_________��
��5���������ʵĺ˴Ź�����������ͼ��ʾ��
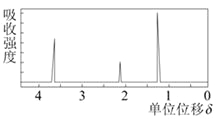
����ṹ��ʽΪ___________________�����л����ͬ���칹��ṹ��ʽΪ ��
���𰸡�
��1������O2
��2���������ӿ�O2������
��3��ʹ�л�������������CO2��H2O��
��4��C2H6O��
��5�� CH3CH2OH�� CH3OCH3
��������
���������ʵ��ԭ���Dzⶨһ���������л�����ȫȼ��ʱ����CO2��H2O����������ȷ���Ƿ�����C��H��O�ĸ����ȣ�������ʽ���ٽ���л�����Է�������ȷ������ʽ��D�����ɵ������к���ˮ������Ӧ��ͨ��C�е�Ũ��������E�е�¯����ʱ�ô�������������Ʒ�����ɶ�����̼��ˮ������һ����̼���ɣ���E��CuO����CO��һ����Ӧ���ɶ�����̼��Ȼ��ֱ�ͨ��B����ˮ���ⶨ����ˮ��������ͨ��A���ն�����̼���ⶨ���ɶ�����̼��������
��1��D�����ɵ������к���ˮ������Ӧ��ͨ��C�е�Ũ����������Ӱ��ʵ�������ʴ�Ϊ������ˮ�ָ���������
��2��MnO2Ϊ�ֽ��Ʊ������Ĵ������ʴ�Ϊ���������ӿ����O2�����ʣ�
��3��һ����̼��������ͭ��Ӧ���ɱ������ɶ�����̼�����ʿ�֪��CuO�������ǰ��л��ﲻ��ȫȼ�ղ�����COת��ΪCO2���ʴ�Ϊ�����л��ﲻ��ȫȼ�ղ�����COת��ΪCO2��
��4��ˮ�����ʵ���=![]() =0.06mol��������̼�����ʵ���=
=0.06mol��������̼�����ʵ���=![]() =0.04mol����m(C)+m(H)=0.04mol��12g+0.06mol��2��1g/mol=0.6g��0.92g�����л��ﺬ��OԪ�أ�m(O)=0.92g-0.6g=0.32g����n(O)=
=0.04mol����m(C)+m(H)=0.04mol��12g+0.06mol��2��1g/mol=0.6g��0.92g�����л��ﺬ��OԪ�أ�m(O)=0.92g-0.6g=0.32g����n(O)=![]() =0.02mol��n(C):n(H):n(O)=0.04:0.12:0.02=2:6:1�����л����ʵ��ʽ��C2H6O������2��Cԭ�������6��Hԭ�ӣ��ʷ���ʽΪC2H6O���ʴ�Ϊ��C2H6O��
=0.02mol��n(C):n(H):n(O)=0.04:0.12:0.02=2:6:1�����л����ʵ��ʽ��C2H6O������2��Cԭ�������6��Hԭ�ӣ��ʷ���ʽΪC2H6O���ʴ�Ϊ��C2H6O��
��5�����л������ʽΪC2H6O�������ʵĺ˴Ź���������3�����շ壬���л����������3��Hԭ�ӣ�������ṹ��ʽΪ��CH3CH2OH�����л����ͬ���칹��ṹ��ʽΪCH3OCH3���ʴ�Ϊ��CH3CH2OH��CH3OCH3��