��Ŀ����
����ʵ�������Ԥ��Ŀ�Ļ����ý���һ�µ���
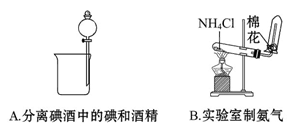
| A���ò�˿պȡij��Һ������ɫ��Ӧ������ʻ�ɫ��֤������Һ��һ��������K�� |
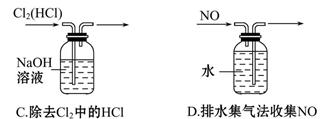
| B����Ũ�Ⱦ�Ϊ0.1 mol��L��1NaCl��NaI�����Һ�еμ�����AgNO3��Һ��������ɫ������˵��Ksp(AgCl)��Ksp(AgI) |
| C����ij��Һ�м��������ữ��Ba(NO3)2��Һ�����ְ�ɫ������˵������Һһ������SO42�� |
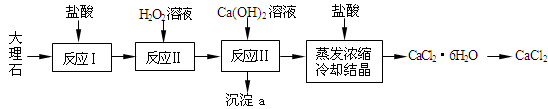
| D�������ᡢ�Ҵ������������Ļ�����м������������ռ���Һ��ֻ�Ϻ��Һ���ɵõ��ϴ������������� |
B
���������A����ɫ��Ӧ�м�Ԫ��Ϊ��ɫ���ܱ���Ԫ�صĻ�ɫ�ڸǣ��ʳʻ�ɫ˵��������Ԫ�أ��������ų���Ԫ�أ�����B��������˵��AgI�����ܣ��ܶȻ���С����ȷ��C������������Ӳ������ţ�����D���ռ�������������Ӧ�����ܷ�����ᴿ�û�������

��ϰ��ϵ�д�
�����Ŀ