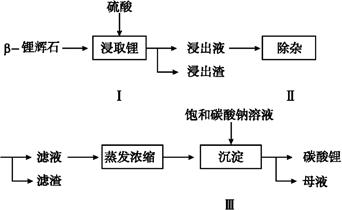
��Ŀ����
��֪ij����Һ��Ag+��Fe2+��Al3+��K+��Ba2+��NH4+��NO3����SO42���е�������������ɣ���������ʵ�飺
��һ�������������ϡ���ᣬ�������ɡ�
�ڶ������������������ϡ���ᣬ�а�ɫ�������ɡ�
�����������ˣ�ȡ������Һ������NaOH��Һ����Һ�ʼ��ԣ�
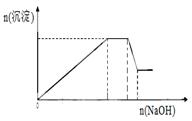
�ڴ˹�������Һ�������ı仯����ͼ��ʾ�����ȸ���Һ��
�ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��������ʵ������ش��������⣺
��1���ô���Һ��һ������ ���ӣ�һ��û�� ���ӣ������� ���ӡ�
��2��ijͬѧ���ò�pH�ķ������жϵ���NaOH��Һ���Ƿ�ʹ��Һ�ʼ��ԣ���ʵ������� ��
��3���������в���ʹʪ���ɫʯ����ֽ����ɫ����������ӷ���ʽΪ ���ò�����Ԥ
�ڻ��������һ�������ʵ�����������Ӧ�Ļ�ѧ����ʽΪ ��

��һ�������������ϡ���ᣬ�������ɡ�
�ڶ������������������ϡ���ᣬ�а�ɫ�������ɡ�
�����������ˣ�ȡ������Һ������NaOH��Һ����Һ�ʼ��ԣ�
�ڴ˹�������Һ�������ı仯����ͼ��ʾ�����ȸ���Һ��
�ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��������ʵ������ش��������⣺
��1���ô���Һ��һ������ ���ӣ�һ��û�� ���ӣ������� ���ӡ�
��2��ijͬѧ���ò�pH�ķ������жϵ���NaOH��Һ���Ƿ�ʹ��Һ�ʼ��ԣ���ʵ������� ��
��3���������в���ʹʪ���ɫʯ����ֽ����ɫ����������ӷ���ʽΪ ���ò�����Ԥ
�ڻ��������һ�������ʵ�����������Ӧ�Ļ�ѧ����ʽΪ ��
��1��Fe2+��Al3+��Ba2+��NH4+��NO3- SO42-��Ag+ K+
��2��ȡһƬpH��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ����Һ������pH��ֽ���в�������Ӻ������ɫ�����գ��ж���Һ�Ƿ�ʼ��ԡ�
��3��NH4++OH- NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3
NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3
��2��ȡһƬpH��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ����Һ������pH��ֽ���в�������Ӻ������ɫ�����գ��ж���Һ�Ƿ�ʼ��ԡ�
��3��NH4++OH-
 NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3
NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3�����������1������HCl��������˵��������Ag+���������������ϡ���ᣬ�а�ɫ��������˵������Ba2+���ӡ������ij�����Ӧ�ķ���ʽΪBa2++ SO42��=BaSO4��������˺����Һ�е���NaOH��Һ����Һ�ʼ������Ȳ����������������ﵽ���ֵ���ٵμӡ����������䣬������ּ���һ���֡�˵��������Fe2+��Al3+��NH4+��������Һ�ʵ����Կ�֪��Һ�л�Ӧ�ú��������ӡ�����Ba2+�� SO42�����ܹ��棬����Ba2+���ӣ�SO42�����ܴ��ڡ���һ��������NO3�����ӡ��ʸô���Һ��һ������Fe2+��Al3+��Ba2+��NH4+��NO3-��һ��������SO42-��Ag+�� K+������Ҳ����û�С���2���ж���Һ�Ƿ�ʼ��ԣ���ʵ�������ȡһƬpH��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ����Һ������pH��ֽ���в�������Ӻ������ɫ�����գ��ж���Һ�Ƿ�ʼ��ԡ���3������ʹʪ���ɫʯ����ֽ����ɫ����������ӷ���ʽΪNH4++OH-
 NH3��+H2O . �ò����п��ܻ��������һ�������ʵ���������Fe(OH)2���ȶ������ױ������е���������ΪFe(OH)3���ῴ�����Ȳ�����ɫ��������������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���������Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2 +O2+ 2H2O=4Fe(OH)3��
NH3��+H2O . �ò����п��ܻ��������һ�������ʵ���������Fe(OH)2���ȶ������ױ������е���������ΪFe(OH)3���ῴ�����Ȳ�����ɫ��������������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���������Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2 +O2+ 2H2O=4Fe(OH)3��
��ϰ��ϵ�д�
�����Ŀ
 ���壬����ֹ����
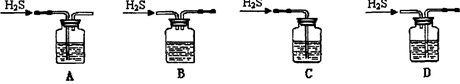
���壬����ֹ���� �������b��a�����������ռ�
�������b��a�����������ռ�
 ���壬�������ȳ��ְ�ɫ������������
���壬�������ȳ��ְ�ɫ������������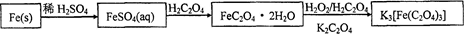
 ��Һ
��Һ
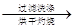 �ù���b g
�ù���b g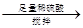
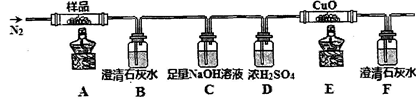
 ��������������Va mL
��������������Va mL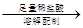 250 mL��Һ
250 mL��Һ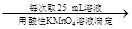 ����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��
����ƽ������0.1 mol��L��1����KMnO4��ҺVb mL����Ϊ���Ϸ����� ��ȷ����Ʒ����ɣ������� ��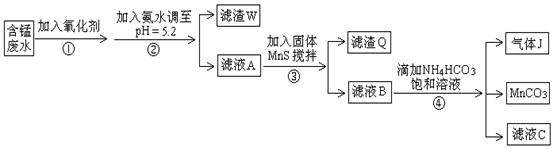
 O2 �� MnO2 + CO2��
O2 �� MnO2 + CO2��