题目内容
(15分)有机物A为芳香烃,质谱分析表明其相对分子质量为92,某课题小组以A为起始原料可以合成酯类香料H和高分子化合物I,其相关反应如下图所示:
已知以下信息:
①碳烯(:CH2)又称卡宾,它十分活跃,很容易用它的两个未成对电子插在烷烃分子的C-H键之间使碳链增长。
②通常在同一个碳原子连有两个羟基不稳定,易脱水形成羰基。
回答下列问题:
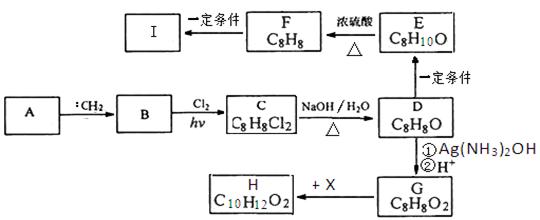
(1)A的化学名称为 。
(2)由B生成C的化学方程式为 ,该反应类型为 。 (3)G的结构简式为 。
(4)请写出由F生成I的化学方程式 。
(5)写出G到H的反应方程式 。
(6)H的所有同分异构体中,满足下列条件的共有 种;
①含有苯环 ②苯环上只有一个取代基 ③属于酯类
其中核磁共振氢谱有五种不同化学环境的氢,且峰面积比为1:1:2:2:6的是 (写结构简式)。
已知以下信息:
①碳烯(:CH2)又称卡宾,它十分活跃,很容易用它的两个未成对电子插在烷烃分子的C-H键之间使碳链增长。
②通常在同一个碳原子连有两个羟基不稳定,易脱水形成羰基。
回答下列问题:

(1)A的化学名称为 。
(2)由B生成C的化学方程式为 ,该反应类型为 。 (3)G的结构简式为 。
(4)请写出由F生成I的化学方程式 。
(5)写出G到H的反应方程式 。
(6)H的所有同分异构体中,满足下列条件的共有 种;
①含有苯环 ②苯环上只有一个取代基 ③属于酯类
其中核磁共振氢谱有五种不同化学环境的氢,且峰面积比为1:1:2:2:6的是 (写结构简式)。
(1)甲苯( )(2)
)(2) ;取代反应
;取代反应
(3) ;(4)
;(4)
(5)
(6)15种,
 )(2)
)(2) ;取代反应
;取代反应(3)
 ;(4)
;(4)
(5)

(6)15种,

试题分析:(1)由于A是芳香烃,结合A的相对分子质量,可确定A是甲苯
 ;(2)根据题意可得B是乙苯
;(2)根据题意可得B是乙苯 ;乙苯与氯气在光照下发生取代反应得到C:
;乙苯与氯气在光照下发生取代反应得到C: 由B生成C的化学方程式为
由B生成C的化学方程式为 ;(3)C与NaOH的水溶液发生反应得到D:
;(3)C与NaOH的水溶液发生反应得到D: ;D与银氨溶液发生反应,然后酸化得到G:
;D与银氨溶液发生反应,然后酸化得到G: ;G与乙醇发生酯化反应得到H:
;G与乙醇发生酯化反应得到H: 。该反应的方程式为
。该反应的方程式为 ;(4)D
;(4)D 与H2发生加成反应得到E:
与H2发生加成反应得到E: ;E在浓硫酸作用下发生消去反应得到F:
;E在浓硫酸作用下发生消去反应得到F: ;F在一定条件下发生加聚反应得到I:
;F在一定条件下发生加聚反应得到I: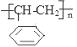 ;反应的方程式是
;反应的方程式是 。(5)G
。(5)G 与乙醇发生酯化反应得到H
与乙醇发生酯化反应得到H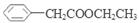 的方程式为
的方程式为 。(6)H的所有符合条件的同分异构体一共有15种,它们分别是:
。(6)H的所有符合条件的同分异构体一共有15种,它们分别是: ;
; ;
; ;
; ;
; ;
; ;
; ;
; ;
; ;
; ;
; ;
;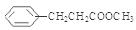 ;
; ;
; ;
; .其中核磁共振氢谱有五种不同化学环境的氢,且峰面积比为1:1:2:2:6的是
.其中核磁共振氢谱有五种不同化学环境的氢,且峰面积比为1:1:2:2:6的是 。
。
练习册系列答案
 能考试期末冲刺卷系列答案
能考试期末冲刺卷系列答案
相关题目
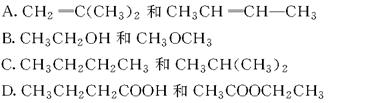
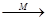 乙酸乙酯,下列说法错误的是
乙酸乙酯,下列说法错误的是
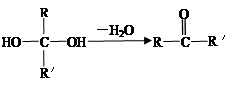
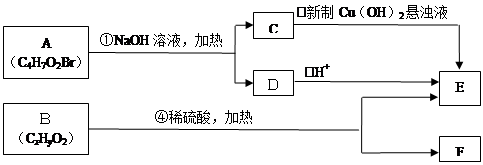
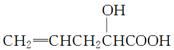 在不同条件下至少可能发生7种不同类型的反应:
在不同条件下至少可能发生7种不同类型的反应: