��Ŀ����
��10�֣�������һ����Ҫ�� ����ԭ�ϣ���ҵ�������������Ҫ�������£�
����ԭ�ϣ���ҵ�������������Ҫ�������£�
��1����N2��H2Ϊԭ�Ϻϳɰ�����һ���¶��£����ܱ������г���ImolN2��3molH2������Ӧ���������ݻ��㶨���ﵽƽ��״̬ʱ������������ʵ�����ԭ����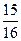 ����N2��ת����
����N2��ת����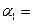 ��������ѹǿ�㶨���ﵽƽ��״̬ʱ��N=��ת����Ϊ
��������ѹǿ�㶨���ﵽƽ��״̬ʱ��N=��ת����Ϊ ����
����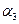
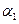 �����>������<����=������
�����>������<����=������
��2���ð������������������ᣬ���������̿ɱ�ʾΪ��
4NH3+5O2 4NO+6H2O 4NO+3O2+2H2O=4HNO3
4NO+6H2O 4NO+3O2+2H2O=4HNO3
����3.4��Һ��Ϊԭ����������������Ϊ50%�����ᣬ��������Ҫ��ˮ������Ϊ
�֡����������������з�Ӧ��������������ģ�
��3�����Ṥҵ�����е�β�����ô�����Һ���գ��йصĻ�ѧ��ӦΪ��
2NO2+Na2CO3=NaNO2+NaNO3+CO2,NO+NO2+Na2CO3=2NaNO2+CO2�����ڱ�״������NO��NO2�Ļ�����壨������N2O4��ǡ����50mL 1�� 0 mol��L-1��Na2CO3��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�Ϊ4��1�����ڻ��������NO������������Ϊ����?����д��������̣�
0 mol��L-1��Na2CO3��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�Ϊ4��1�����ڻ��������NO������������Ϊ����?����д��������̣�
 ����ԭ�ϣ���ҵ�������������Ҫ�������£�
����ԭ�ϣ���ҵ�������������Ҫ�������£���1����N2��H2Ϊԭ�Ϻϳɰ�����һ���¶��£����ܱ������г���ImolN2��3molH2������Ӧ���������ݻ��㶨���ﵽƽ��״̬ʱ������������ʵ�����ԭ����
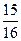 ����N2��ת����
����N2��ת����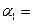 ��������ѹǿ�㶨���ﵽƽ��״̬ʱ��N=��ת����Ϊ
��������ѹǿ�㶨���ﵽƽ��״̬ʱ��N=��ת����Ϊ ����
����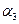
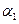 �����>������<����=������
�����>������<����=��������2���ð������������������ᣬ���������̿ɱ�ʾΪ��
4NH3+5O2
 4NO+6H2O 4NO+3O2+2H2O=4HNO3
4NO+6H2O 4NO+3O2+2H2O=4HNO3����3.4��Һ��Ϊԭ����������������Ϊ50%�����ᣬ��������Ҫ��ˮ������Ϊ
�֡����������������з�Ӧ��������������ģ�
��3�����Ṥҵ�����е�β�����ô�����Һ���գ��йصĻ�ѧ��ӦΪ��
2NO2+Na2CO3=NaNO2+NaNO3+CO2,NO+NO2+Na2CO3=2NaNO2+CO2�����ڱ�״������NO��NO2�Ļ�����壨������N2O4��ǡ����50mL 1��
 0 mol��L-1��Na2CO3��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�Ϊ4��1�����ڻ��������NO������������Ϊ����?����д��������̣�
0 mol��L-1��Na2CO3��Һ��Ӧ��ȫ��������NaNO2��NaNO3�����ʵ����ı�Ϊ4��1�����ڻ��������NO������������Ϊ����?����д��������̣� ��1��12��5����1��8��>��4�֣�ÿ��2�֣� ��2��9��3�֣�
��2���������ɷ�Ӧ4NH3+5O2

NH3+2O2=HNO3+H2O��3.4��Һ������������Ϊ12.6�֣�ˮΪ3.6�֣������������ˮ������ΪΪ��126.t/50%-(12.6+3.6)t=9t
��3����3�֣��ɷ�Ӧת����ϵ֪����Ӧ��������Ԫ��
 �غ㣬��NaNO2��NaNO3�����ʵ����ֱ�Ϊ4a mol��a mol����4a+a=50mL��10-3mL��L-1��1��0 mol��L-1��2����ã�a=0��02 mol�����ǣ��Ƴ�NO��NO2�����ʵ�������Ϊ��0��03 mol��0��07 mol������NO��ռ���������Ϊ30����
�غ㣬��NaNO2��NaNO3�����ʵ����ֱ�Ϊ4a mol��a mol����4a+a=50mL��10-3mL��L-1��1��0 mol��L-1��2����ã�a=0��02 mol�����ǣ��Ƴ�NO��NO2�����ʵ�������Ϊ��0��03 mol��0��07 mol������NO��ռ���������Ϊ30������

��ϰ��ϵ�д�
�����Ŀ
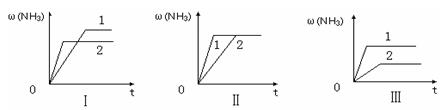
 ��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
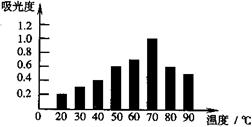
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________�� ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
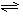 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�����������ҡ���������ʹ�ô��� ��Ӧ�Ħ�H���������С�����ı䡱����
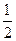 H2��g��+
H2��g��+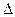 H="_____________" kJ��mol-1��
H="_____________" kJ��mol-1�� A + B + C
A + B + C
 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺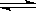 H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K