��Ŀ����
��8�֣��������ʵ�����A��B�����1 L���ܱ������У��������·�Ӧ
3A(g)��B(g)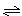 xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
(1)��ʱA��Ũ��c(A)��________mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
(2)B��ƽ����Ӧ����v(B)��________mol/(L��min)��
(3)x��ֵΪ________��
3A(g)��B(g)
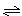 xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����(1)��ʱA��Ũ��c(A)��________mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
(2)B��ƽ����Ӧ����v(B)��________mol/(L��min)��
(3)x��ֵΪ________��
(1)0.75��1.5 (2)0.05 (3) 2 ��ÿ��2�֣�
������淴Ӧ���йؼ��㡣
��1��C��ƽ����Ӧ������0.1mol/(L��min)����C��Ũ����0.5mol/L�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪x��2������Ϊ��Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȣ�����B�ķ�Ӧ������0.1mol/(L��min)��2��0.05mol/(L��min)��
��2�� 3A��g��+B(g)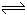 2C(g)+2D(g)
2C(g)+2D(g)
��ʼ����mol�� n n 0 0
ת������mol�� 0.5 0.25 0.5 0.5
ƽ������mol�� n��0.75 n��0.25 0.5 0.5
������n��0.75���U��n��0.25���� 3:5
���n��1.5mol������A��Ũ����0.75mol��1L��0.75mol/L
��1��C��ƽ����Ӧ������0.1mol/(L��min)����C��Ũ����0.5mol/L�����Ը��ݱ仯��֮������Ӧ�Ļ�ѧ������֮�ȿ�֪x��2������Ϊ��Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȣ�����B�ķ�Ӧ������0.1mol/(L��min)��2��0.05mol/(L��min)��
��2�� 3A��g��+B(g)
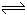 2C(g)+2D(g)
2C(g)+2D(g)��ʼ����mol�� n n 0 0
ת������mol�� 0.5 0.25 0.5 0.5
ƽ������mol�� n��0.75 n��0.25 0.5 0.5
������n��0.75���U��n��0.25���� 3:5
���n��1.5mol������A��Ũ����0.75mol��1L��0.75mol/L

��ϰ��ϵ�д�
�����Ŀ
 2XY2
2XY2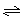 C��g���Ѵﵽƽ��״̬���� �� ��
C��g���Ѵﵽƽ��״̬���� �� �� pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С���� 2NH3��g����H=" ��92.4" kJ/mol��
2NH3��g����H=" ��92.4" kJ/mol�� N2O4�ﵽƽ��ı�־��(����)
N2O4�ﵽƽ��ı�־��(����) pC�Ŀ��淴Ӧ�ﵽƽ�⣺
pC�Ŀ��淴Ӧ�ﵽƽ�⣺