��Ŀ����
SO2ͨ�����й������̿��ƻ���ҵԭ��H2SO4�������ԴH2��
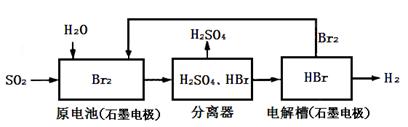
����˵������ȷ����

����˵������ȷ����
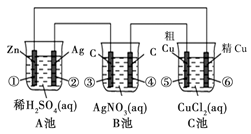
| A��������Ҳ���������缫����ʯī��Ϊ���� |
| B��ԭ����и����ĵ缫��ӦΪ��SO2+2H2O+4e-=SO42-+4H+ |
| C���ù����������ܷ�Ӧ�Ļ�ѧ����ʽ��ʾΪ��SO2+Br2+2H2O=2HBr+H2SO4 |
| D�����������յ��ŵ�Br2��ѭ�����ã�ԭ��ز����ĵ��ܿɳ�����ã����ܻ�������Դ |
D
���������A���������缫���棬����ʧ���ӣ�����B����ɲ��غ㣬����C���ܷ�ӦΪ:
SO2+2H2O=H2+H2SO4������D��������ͼ��֪�������ѭ��ʹ�ã�������Ⱦ�Ե��������ɡ�

��ϰ��ϵ�д�
�����Ŀ

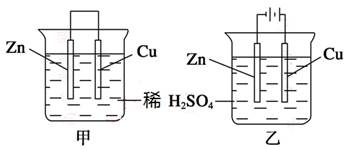
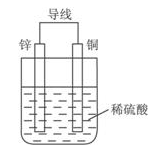
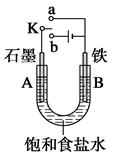

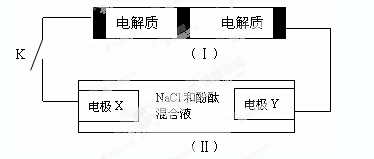
 Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����
Na2S4+3NaBr�����պϿ���Kʱ��X�缫������Һ��졣����˵����ȷ����