��Ŀ����
20�� ����Ԫ�������γɶ��������磺[Fe��CN��6]4-��Fe��SCN��3�ȣ�
����Ԫ�������γɶ��������磺[Fe��CN��6]4-��Fe��SCN��3�ȣ���1��Fe��̬��������Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2��
��2����ѧ�о�������TiO2��������ɽ���ˮ��CN-ת��ΪOCN-������������ΪN2��CO2��OCN-������Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��C��
��3����OCN-��Ϊ�ȵ������һ�ַ���ΪCO2���ѧʽ��
��4����Ԫ�ػ�����һЩ�������γ������Ȼ���̼ԭ�ӵ��ӻ�������sp2��1mol�����к��ЦҼ�����ĿΪ7mol��
��5�������Ͻ��һ�־����������������ṹ���侧���ɿ�����8��С���������ṹ�������ɣ���֪С��������ͼ��ʾ���úϽ�Ļ�ѧʽΪAlFe3��
���� ��1����������ԭ������Ϊ26������������ԭ����д��̬ԭ�ӵĵ����Ų�ʽ��
��2��ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�����IIA��͵�VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��3��ԭ�Ӹ�������Ҽ۵�������ȵķ��ӻ�����Ϊ�ȵ����壻
��4���Ȼ���̼ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6}{2}$=3���ݴ��ж�̼ԭ���ӻ����ͣ���������Ľṹ��ʽCH3COOH��֪��1mol�����к���7mol���ۼ����ݴ��жϦҼ�����Ŀ��
��5�������������У������ϵ�ԭ�ӱ�8������ռ�У������ϵ�ԭ�ӱ�1������ռ�У������Ͻ��ṹ��ԭ�ӵ���ĿΪ4��$\frac{1}{8}$=$\frac{1}{2}$����ԭ����ĿΪ1+4��$\frac{1}{8}$=$\frac{3}{2}$��������ԭ������ԭ�Ӹ���֮��Ϊ1��3���ݴ�ȷ����ѧʽ��
��� �⣺��1������ԭ������Ϊ26�������������ԭ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2��
�ʴ�Ϊ��1s22s22p63s23p63d64s2��[Ar]3d64s2��
��2��ͬ���ڵ�һ������������Ҿ����������ƣ����Ե�һ������O��C�����ڵ�Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�أ�����C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳����N��O��C��
�ʴ�Ϊ��N��O��C��
��3��OCN-����3��ԭ�ӣ��۵�������Ϊ6+4+5+1=16������ȵ�����ΪCO2�ȣ�
�ʴ�Ϊ��CO2��
��4���Ȼ���̼ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6}{2}$=3������̼ԭ���ӻ�����Ϊsp2�ӻ�����������Ľṹ��ʽCH3COOH��֪��1mol�����к���7mol���ۼ���ÿ�����ۼ��ж�����1���Ҽ�������1mol�����к��ЦҼ�����ĿΪ7mol��
�ʴ�Ϊ��sp2��7mol��
��5�����������Ͻ��ṹ��ԭ�ӵ���ĿΪ4��$\frac{1}{8}$=$\frac{1}{2}$����ԭ����ĿΪ1+4��$\frac{1}{8}$=$\frac{3}{2}$��������ԭ������ԭ�Ӹ���֮��Ϊ1��3���������Ͻ�Ļ�ѧʽΪ��AlFe3��
�ʴ�Ϊ��AlFe3��
���� ���⿼���Ϊ�ۺϣ��漰�����Ų�ʽ���縺�ԡ��Ҽ����Լ�����ṹ������֪ʶ����Ŀ�Ѷ��еȣ�ע��ͬһ�����е�һ�����ܵı仯���Ƽ��쳣����Ϊ�״��㣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 4HCl+MnO2$\frac{\underline{\;����\;}}{\;}$MnCl2+Cl2��+2H2O | |
| B�� | 2HCl+CaCO3=CaCl2+CO2+H2O | |
| C�� | 2HCl+Zn=ZnCl2+H2�� | |
| D�� | HCl+AgNO3=AgCl��+HNO3 |
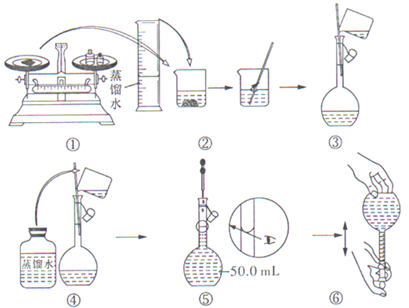
��1������250mL0.1mol/L��������Һ
| Ӧ��ȡ�������/mL | Ӧѡ������ƿ�Ĺ��/mL |
| 2.1mL | 250mL |
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳ��ȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2-3cm��
��3����Һע������ƿǰ��ָ������£�������Ϊ����ƿ�������ȣ������������
��4����ע������ƿǰδ�ָ������£���������ҺŨ�Ƚ��к�Ӱ�죿ƫ�ߣ��ƫ�͡���ƫ�ߡ�����Ӱ�족��
| A�� | ��״̬ʱ��22.4L��CO2 | B�� | 200g49%��H3PO4 | ||
| C�� | 32g ��SO2���� | D�� | 3.01��1023��O2���� |