��Ŀ����
����Ŀ���ҹ�ũҵ�����������������ɵ���ʧÿ��ߴ�15��Ԫ��Ϊ����Ч�������꣬Ŀǰ����Ժ�����ˡ�����������Ͷ���������Ⱦ���������ַ������ȷ��档ijʵ��С��ɼ�������������Ʒ������ʱ������ƣ���βⶨ����Ʒ��pH���õ��˱������ݣ�(��֪pHԽС����Һ������Խǿ)
ʱ��(h) | 0 | 8 | 16 | 24 | 32 | 40 | 48 |
pH | 5.0 | 4.8 | 4.5 | 4.3 | 4.2 | 4.0 | 4.0 |
(1)������Ʒ����ʱpH�仯����Ҫԭ����(�û�ѧ����ʽ��ʾ)____________________��
(2)�������ȡ�����������������ˮ��ϣ�pH��________(�������С�����䡱)��ԭ����(�û�ѧ����ʽ��ʾ)____________________��
(3)����Ϊ������������ɲ�ȡ�Ĵ�ʩ��___________(����ĸ)��
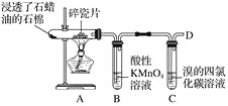
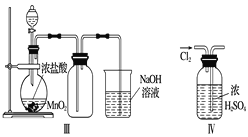
������ú��ȼ�ϡ��ڰѹ����̴���ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
A���٢ڢ� B���ڢۢܢ� C���٢ۢ� D���٢ۢܢ�
���𰸡� SO2+H2O![]() H2SO3��2H2SO3+O2===2H2SO4 ��С H2SO3+Cl2+H2O===2HCl+H2SO4 C
H2SO3��2H2SO3+O2===2H2SO4 ��С H2SO3+Cl2+H2O===2HCl+H2SO4 C
��������
(1)�������ʱpH��С����ԭ������ˮ�е�H2SO3�������е�O2�������¡�
(2)��ˮ������ˮ��ϣ�Ӧ����H2SO3������ˮ�е�Cl2����������ԭ��Ӧ��������������ᣬʹ������ǿ��
(3)����������γɱ������SO2�ŷţ���úת��Ϊ�����Դ(��ú��������Һ��)��ȼ���������������������Դ(�����ܡ����ܵ�)��������Ч��ʩ��

 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�����Ŀ�����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�˻������ú��ִ���(V2O5��VOSO4������������)��������Ա����������һ�����ӽ��������շ����¹��ա��ù��յ���Ҫ������ͼ��ʾ:
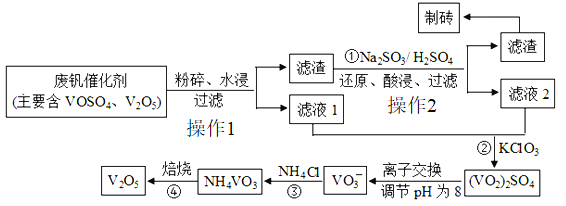
��֪:���ֺ���������ˮ�е��ܽ���:
���� | V2O5 | VOSO4 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
(1)��V2O5ұ���������������ȼ�������Ӧ�Ļ�ѧ����ʽΪ:___________
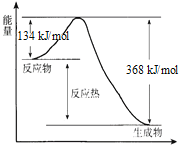
(2)V2O5ͨ������Ϊ��Ӧ2SO2+O2![]() 2SO3���������������Ļ��������Ǽ���ʱ�����ʧȥ����ԭ������̿���������ѧ����ʽ��ʾ:_________��4V+5O2
2SO3���������������Ļ��������Ǽ���ʱ�����ʧȥ����ԭ������̿���������ѧ����ʽ��ʾ:_________��4V+5O2![]() 2V2O5.
2V2O5.
(3)��Ӧ�ٵ�Ŀ����_________.
(4)����Һ1����Һ2��Ϻ����������������Ԫ�ر���ԭΪ��ͼۣ��䷴Ӧ�����ӷ���ʽΪ______.
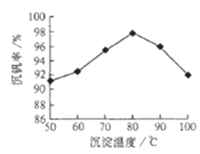
(5)��Ӧ�۵ij�����(�ֳƳ�����) �ǻ��շ��Ĺؼ�֮һ����ͼ�Ƿ�Ӧ�¶�������ʵĹ�ϵͼ��������¶ȵķ�����____________.
(6)��Ӧ����NH4VO3�ı��չ����У����������ļ���ֵ(������) ���¶ȱ仯��������ͼ2��ʾ������ֽ������_______(����ĸ����)��
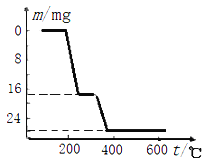
A.�ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3
B.�ȷֽ�ʧȥNH3�� �ٷֽ�ʧȥH2O
C.ͬʱ�ֽ�ʧȥH2O ��NH3
D.ͬʱ�ֽ�ʧȥH2��N2��H2O
(7)ȫ����صĵ������ҺΪVOSO4��Һ����صĹ���ԭ��ΪVO2++V2++2H+![]() VO2++H2O+V3+����س��ʱ�����ĵ缫��ӦʽΪ__________
VO2++H2O+V3+����س��ʱ�����ĵ缫��ӦʽΪ__________