��Ŀ����
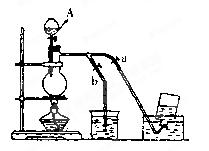
��15�֣���������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�װ����ͼ9��
��֪��Na2S2O3��������Һ�в����ȶ����ڣ��й����ʵ��ܽ��������ͼ10��ʾ��

��1��Na2S2O3��5H2O���Ʊ���
����1����K1���ر�K2����Բ����ƿ�м�������Ũ���Ტ���ȡ�д����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ�� ��
����2��C�л��Һ��������������Ӧһ��ʱ�����۵������١���C����Һ��pH ʱ����K2���ر�K1��ֹͣ���ȣ������� ��װ��B��D�������� ��
����3������C�еĻ��Һ������Һ���� �� �����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2��Na2S2O3���ʵļ��飺
��������ˮ�м���Na2S2O3��Һ����ˮ��ɫ��dz��������Һ�еμ���������Һ���۲쵽�а�ɫ�����������ݴ���ΪNa2S2O3���л�ԭ�ԡ��÷����Ƿ���ȷ��˵������ ��
��3������Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȣ��������£�ȡ��ˮ25.00 mL�������ʵ�����ȼ�������K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټӹ���KI��Һ����ַ�Ӧ���������Һ��ָʾ������0.010 mol��L��1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ������Na2S2O3��Һ18.00 mL�����ַ�Ӧ�����ӷ���ʽΪ��Cr2O72��+ 6I��+ 14H+ 2Cr3++3I2+7H2O����I2+ 2S2O32�� 2I��+ S4O62����
��÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ ��
��֪��Na2S2O3��������Һ�в����ȶ����ڣ��й����ʵ��ܽ��������ͼ10��ʾ��

��1��Na2S2O3��5H2O���Ʊ���
����1����K1���ر�K2����Բ����ƿ�м�������Ũ���Ტ���ȡ�д����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ�� ��
����2��C�л��Һ��������������Ӧһ��ʱ�����۵������١���C����Һ��pH ʱ����K2���ر�K1��ֹͣ���ȣ������� ��װ��B��D�������� ��
����3������C�еĻ��Һ������Һ���� �� �����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2��Na2S2O3���ʵļ��飺
��������ˮ�м���Na2S2O3��Һ����ˮ��ɫ��dz��������Һ�еμ���������Һ���۲쵽�а�ɫ�����������ݴ���ΪNa2S2O3���л�ԭ�ԡ��÷����Ƿ���ȷ��˵������ ��
��3������Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȣ��������£�ȡ��ˮ25.00 mL�������ʵ�����ȼ�������K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټӹ���KI��Һ����ַ�Ӧ���������Һ��ָʾ������0.010 mol��L��1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ������Na2S2O3��Һ18.00 mL�����ַ�Ӧ�����ӷ���ʽΪ��Cr2O72��+ 6I��+ 14H+
��÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ ��
��15�֣�
��1��Cu + 2H2SO4(Ũ)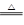 CuSO4 + 2H2O + SO2�� ��2�֣�
CuSO4 + 2H2O + SO2�� ��2�֣�
�ӽ�7 ��1�֣� Na2S2O3��������Һ�в����ȶ����ڣ�2�֣�
����SO2����ֹ��Ⱦ ��2�֣�
����Ũ������ȴ�ᾧ ��ÿ��2�֣�
��2������ȷ����Ϊ��ˮ�к���Cl�� ��2�֣�
��3��2.4��10��3 mol��L��1 ��2�֣�
��1��Cu + 2H2SO4(Ũ)
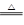 CuSO4 + 2H2O + SO2�� ��2�֣�
CuSO4 + 2H2O + SO2�� ��2�֣��ӽ�7 ��1�֣� Na2S2O3��������Һ�в����ȶ����ڣ�2�֣�
����SO2����ֹ��Ⱦ ��2�֣�
����Ũ������ȴ�ᾧ ��ÿ��2�֣�
��2������ȷ����Ϊ��ˮ�к���Cl�� ��2�֣�
��3��2.4��10��3 mol��L��1 ��2�֣�
��1������1��ͭ�ڼ��ȵ������¿ɻ�ԭŨ���ᣬ����SO2��Cu + 2H2SO4(Ũ) 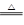 CuSO4 + 2H2O + SO2��
CuSO4 + 2H2O + SO2��
����2��SO2��S��Na2CO3=Na2S2O3+CO2������Na2S2O3��������Һ�в����ȶ����ڣ�ԭ��Һˮ��ʼ��ԣ�����SO2��ͨ�룬������������pH�ӽ�7ʱ��ֹֹͣͣͨ��������ֹͣ����
B��Dװ�þ���Ϊ������SO2����ֹ��Ⱦ
����3��Na2S2O3���ܽ�����¶Ƚ��Ͷ�Ѹ�ټ�С��Na2CO3���ܽ�����¶ȱ仯��С����Ϊ�˵õ�Na2S2O3��������Ũ������ȴ�ᾧ���ٹ��ˡ�ϴ�ӡ�������ȡ��Ʒ
��2��������ˮ�к���Cl����Cl2��H2O=H����Cl����HClO���������м���AgNO3��Һ����ˮ��ɫ��dz���а�ɫ�������ɣ�������˵��Na2S2O3��ԭ�����������л�ԭ��
��3���ɹ�ϵʽBa2+��BaCrO4��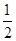 CrO42����
CrO42����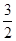 I2��3Na2S2O3��֪��n(Ba2+)=
I2��3Na2S2O3��֪��n(Ba2+)=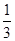 ��0.010��
��0.010��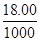 =0.0006mol��c(Ba2+)=2.4��10��3 mol��L��1
=0.0006mol��c(Ba2+)=2.4��10��3 mol��L��1
 CuSO4 + 2H2O + SO2��
CuSO4 + 2H2O + SO2�� ����2��SO2��S��Na2CO3=Na2S2O3+CO2������Na2S2O3��������Һ�в����ȶ����ڣ�ԭ��Һˮ��ʼ��ԣ�����SO2��ͨ�룬������������pH�ӽ�7ʱ��ֹֹͣͣͨ��������ֹͣ����
B��Dװ�þ���Ϊ������SO2����ֹ��Ⱦ
����3��Na2S2O3���ܽ�����¶Ƚ��Ͷ�Ѹ�ټ�С��Na2CO3���ܽ�����¶ȱ仯��С����Ϊ�˵õ�Na2S2O3��������Ũ������ȴ�ᾧ���ٹ��ˡ�ϴ�ӡ�������ȡ��Ʒ
��2��������ˮ�к���Cl����Cl2��H2O=H����Cl����HClO���������м���AgNO3��Һ����ˮ��ɫ��dz���а�ɫ�������ɣ�������˵��Na2S2O3��ԭ�����������л�ԭ��
��3���ɹ�ϵʽBa2+��BaCrO4��
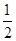 CrO42����
CrO42����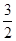 I2��3Na2S2O3��֪��n(Ba2+)=
I2��3Na2S2O3��֪��n(Ba2+)=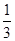 ��0.010��
��0.010��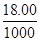 =0.0006mol��c(Ba2+)=2.4��10��3 mol��L��1
=0.0006mol��c(Ba2+)=2.4��10��3 mol��L��1
��ϰ��ϵ�д�
 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
�����Ŀ
 ֤�������ʵ�װ��ͼ
֤�������ʵ�װ��ͼ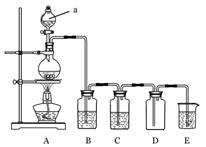
 ƫ�ߡ���ƫ�͡�����Ӱ�족����
ƫ�ߡ���ƫ�͡�����Ӱ�족����
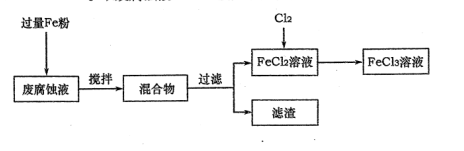
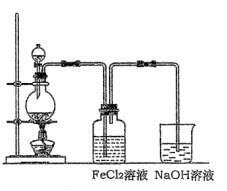
 ��
��