��Ŀ����
�ڶ���������Ԫ����
��1�����ڷǽ���Ԫ�ص��־���һ���Ľ����ԣ������뵼����ϵ���________����Ԫ�����ƣ���
��2���ס�������Ԫ����ͬһ���ڵ�����Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ���Ԫ�ص�L������5�����ӡ��ס���Ԫ�ؿ��γ�Ӳ�ȴ��ڽ��ʯ��һ�ֻ�����û���������____________���壬�仯ѧʽΪ_______________���ϳ��������ʱ���ķ�Ӧ������ ��
��3����ȥ���˱�������Ĥ�������������ṯ��Һ��Ƭ�̺�ȡ��������һ��ʱ��ɿ��������������ɰ�ɫë״����ɸ����ʵĻ�ѧ����ʽΪ_______________________________��������ѧ�ҷ������ġ�ԭ��Ⱥ������Al13��Al14��Al37����������ij��Ԫ�����ƵĻ�ѧ���ʣ�������Ԫ�������ɵ�ijЩ���ɡ����磺Al13��±�ؾ������ƵĻ�ѧ���ʣ� Al13�����Ӿ��ж�������Ԫ�ص����ʣ����Ʋ�Al14�Ļ�ѧ������ ��Ԫ�����ơ�
��1���裨2��ԭ�� ��C3N4 �����¡���ѹ ��3��4Al + 3O2 = 2Al2O3 IIA
����:
���⿼��Ԫ�����ڱ�����1��������Ϊ�뵼��ij������ϣ���2����Ԫ��Ϊ��Ԫ�أ���Ԫ�����γ��л������ҪԪ�أ�Ϊ̼Ԫ�أ��ס���Ԫ���γɻ�����ΪC3N4����3�����������е�����������Al13�����Ӿ��ж�������Ԫ�ص����ʣ�Ϊ�ȶ��ṹ����ôAl14�൱�ڱ�Al13������2�����ӣ���IIAԪ���������ơ�

 ������������ϵ�д�
������������ϵ�д�

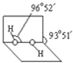 ��4����֪����������ӵĿռ�ṹ��ͼ��ʾ����������ԭ�Ӳ�ȡ
��4����֪����������ӵĿռ�ṹ��ͼ��ʾ����������ԭ�Ӳ�ȡ
