��Ŀ����
����Ŀ������ͭ���壨CuSO4��xH2O����һ����;�㷺���Լ���ijС����̽������ͭ��������ʣ����ⶨ��ᾧˮ������
ʵ�飨һ����̽������ͭ�������ԡ�
ȡ��������ͭ��Һ���Թ��У�����(NH4)2SO3��Һ����������M�����ˡ�ϴ�ӣ��õ�����M��Ϊ��̽��M����ɣ���������ʵ�飺
�ٽ�һ��������M�ֳ����ݡ�
����һ�ݹ����м���ϡ���ᣬ�����д̼�����ζ������(X)����Һ�����ɫ���к�ɫ�������ɣ�������ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ��
������һ�ݹ����м���Ũ�ռ���Һ�����ȣ���������(Y)����������ʹʪ��ĺ�ɫʯ����ֽ������
�ش��������⣺
��1��Y�ĵ���ʽΪ___________��
��2�����ⶨM�������ӡ������Ӹ���֮��Ϊ2��1��M�Ļ�ѧʽΪ______________��
ʵ�飨������̽������ͭ��������ȶ��ԡ�
ȡ��������ͭ�������ʵ�飬װ����ͼ��ʾ��
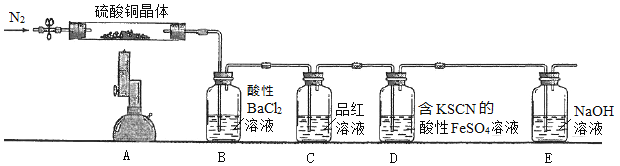
��֪����ʵ������Ϊ��A����ɫ������ɰ�ɫ��ĩ������ɺ�ɫ��ĩ��B�в�����ɫ������D����Һ��ɺ�ɫ��
��3�������Ʋ�����ͭ����ķֽ������________________________________��
��4��B��Cװ�õ�λ�ò��ܻ�����ԭ����_______________________________��
��5��D�еķ�Ӧ���������У�д����һ����Ӧ�����ӷ���ʽ___________________________��
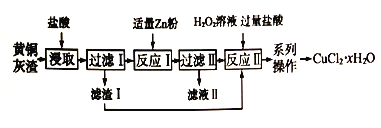
ʵ�飨�������ⶨ����ͭ�����нᾧˮ�ĺ�����
ȡwg����ͭ���壨CuSO4��xH2O�����250 mL ��Һ��ȡ25.00 mL ��Һ��c mol/L EDTA��Һ(��д��Y4��)�ζ����յ㣬����EDTA��ҺV mL��
��֪���ζ�����ʽΪ��Cu2++ Y4��=CuY2����
��6��x=___________________���ô���ʽ��ʾ����
��7�����������ʹ��õ�xƫС����_______����ţ�
a����Ʒʧȥ���ֽᾧˮ
b����ȡ����Һǰδ�ô���Һ��ϴ�ζ���
c����ʼ����ʱ�ζ��ܼ��������ݶ��յ�ʱ������
d���ζ���ʼʱƽ�ӡ��ζ��յ�ʱ����
���𰸡� ![]() NH4CuSO3 H2O��CuO��SO3��SO2��O2 ������SO3����Ʒ����Һ��BaCl2��Һ�������SO3 4Fe2++O2+4H+=4Fe3++2H2O (50w��80cV)/9cV ac
NH4CuSO3 H2O��CuO��SO3��SO2��O2 ������SO3����Ʒ����Һ��BaCl2��Һ�������SO3 4Fe2++O2+4H+=4Fe3++2H2O (50w��80cV)/9cV ac
��������(1). ����������Ϣ��֪��ȡ��������ͭ��Һ���Թ��У�����(NH4)2SO3��Һ����������M��M�м���Ũ�ռ���Һ�����ȣ���������(Y)����������ʹʪ��ĺ�ɫʯ����ֽ������˵������(Y)ΪNH3��M�к���笠����ӣ�NH3�ĵ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2). M��ϡ���ᷴӦʱ����Һ�����ɫ���к�ɫ����������˵��M�к���Cu+��Cu+����������������Cu��Cu2���������д̼�����ζ������(X)��������ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ��˵�����ɵ�����XΪSO2����M�к���SO32����HSO3��������M�������ӡ������Ӹ���֮��Ϊ2:1����M�к���SO32����M�Ļ�ѧʽΪ��NH4CuSO3���ʴ�Ϊ��NH4CuSO3��
(3). ��ʵ��װ��ͼ��֪���ڼ�������ͭ����ǰ��ͨ�뵪�������Խ�����װ���еĿ����ϳ���Ȼ���������ͭ���壬A����ɫ������ɰ�ɫ��ĩ��˵��ʧȥ�ᾧˮ������ɺ�ɫ��ĩ��˵��������CuO��B��ʢ�������Ȼ�����Һ��B�в�����ɫ������˵��������SO3��D��ʢ�к�KSCN������FeSO4��Һ��D����Һ��ɺ�ɫ��˵��Fe2+������������Fe3+��������ͭ����ķֽ�����к���O2������ͭ�����е�OԪ�ػ��ϼ۴���2�����ߵ�0����˵��SԪ�صĻ��ϼ۽���������SO2���������ͭ����ķֽ��������H2O��CuO��SO3��SO2��O2���ʴ�Ϊ��H2O��CuO��SO3��SO2��O2��
(4). ��SO3��������ˮ��������SO3����Ʒ����Һ����BaCl2��Һ�������SO3���ʴ�Ϊ��������SO3����Ʒ����Һ��BaCl2��Һ�������SO3��
(5). �����������£�����ͭ����ֽ����ɵ�������Fe2+������Fe3+��Fe3+����SCN����Ӧ����Fe(SCN)3����һ����Ӧ�����ӷ���ʽΪ��4Fe2++O2+4H+=4Fe3++2H2O���ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O��
(6). �ɵζ�����ʽCu2++ Y4��=CuY2����֪��n(CuSO4)=V��10��3L��c mol/L��![]() =Vc��10��2mol����n(H2O)=
=Vc��10��2mol����n(H2O)= ![]() ��x=
��x=![]() =(50w��80cV)/9cV���ʴ�Ϊ��(50w��80cV)/9cV��
=(50w��80cV)/9cV���ʴ�Ϊ��(50w��80cV)/9cV��
(7). A.��Ʒʧȥ���ֽᾧˮ����ʹ�ⶨ�Ľᾧˮ����ƫ����xƫС����a��ȷ��b.��ȡ����Һǰδ�ô���Һ��ϴ�ζ�������ʹ���ĵı�Һ���ƫ�٣��ⶨ�Ľᾧˮ����ƫ����xƫ��b������c.��ʼ����ʱ�ζ��ܼ��������ݶ��յ�ʱ�����ݣ���ʹ��ȡ�ı�Һ���ƫ�ⶨ�Ľᾧˮ����ƫ�٣�xƫС����c��ȷ��d.�ζ���ʼʱƽ�ӡ��ζ��յ�ʱ��������ʹ��ȡ�ı�Һ���ƫС���ⶨ�Ľᾧˮ����ƫ��xƫ��d����ѡ��ac��

����Ŀ���±��ж����ӷ���ʽ�����ۺ�������
ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
A | H2SO4��Һ��Ba(OH)2��Һ��Ӧ�� H++ SO42-+Ba2++OH | ��ȷ |
B | ��̼��þ��Һ�м�������ϡ��� CO32-+2H+ | ����̼��þ��Ӧд��������ʽ |
C | ���ˮ�еμӱ��͵��Ȼ�����Һ��Һ���Ϊ���ɫ��Fe3++3H2O | ��ȷ |
D | ��NaOH��Һ��ͨ������CO2��Ӧ��OH+CO2 | ��ȷ |
A. A B. B C. C D. D