��Ŀ����
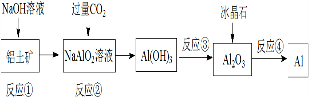
9���ɶ�����Ԫ����ɵ����ֵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾת����ϵ�����ǵ��͵����������Ҳ�ǹ�ҵ����ȡA����Ҫԭ�ϣ�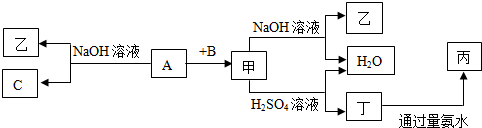
��ش�
��1��д��NH3�ĵ���ʽ��
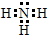 ��
����2��д���Ļ�ѧʽ��Al2O3��
��3��д��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��4��д�����������ˮ��Ӧ�����ӷ���ʽ��Al3++3NH3��H2O=Al��OH��3��+3NH4+��
���� ���ǵ��͵�����������������ᷴӦ������Ӧ�����ΪAl2O3�������������Ʒ�Ӧ�����ң�����ΪNaAlO2����Ҳ�ǹ�ҵ����ȡA����Ҫԭ�ϣ���A������NaOH��Ӧ�����Һ�C����CΪH2��AΪAl��A��B��Ӧ��������������BΪO2���������ᷴӦ���ɶ�ΪAlCl3�����������ˮ��Ӧ���ɱ�ΪAl��OH��3���ݴ˽��
��� �⣺���ǵ��͵�����������������ᷴӦ������Ӧ�����ΪAl2O3�������������Ʒ�Ӧ�����ң�����ΪNaAlO2����Ҳ�ǹ�ҵ����ȡA����Ҫԭ�ϣ���A������NaOH��Ӧ�����Һ�C����CΪH2��AΪAl��A��B��Ӧ��������������BΪO2���������ᷴӦ���ɶ�ΪAlCl3�����������ˮ��Ӧ���ɱ�ΪAl��OH��3��
��1��NH3�ĵ���ʽΪ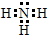 ���ʴ�Ϊ��
���ʴ�Ϊ��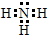 ��
��
��2���Ļ�ѧʽΪ��Al2O3���ʴ�Ϊ��Al2O3��
��3��AΪAl��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��4�����������ˮ��Ӧ�����ӷ���ʽ��Al3++3NH3��H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3��H2O=Al��OH��3��+3NH4+��
���� ���⿼�������ƶϣ��ѶȲ�����Ҫѧ���������������仯��������֪ʶ���Ƚϻ������漰����ʽ����ѧ����ʽ�����ӷ�Ӧ����ʽ�ȣ�����ʱע�⻯ѧ����Ĺ淶��д��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �μӹ�����ˮ�ĵ���̶�ʼ������ | |
| B�� | ������10mL NH3•H2Oʱ��c��NH${\;}_{4}^{+}$����c��HCOO-�� | |
| C�� | ������ǡ���к�ʱ����ҺpH=7 | |
| D�� | �μӹ�����c��HCOOH����c��HCOO-��֮�ͱ��ֲ��� |
| A�� | �ں��ִ���������п�鱣�����֣����õ����������������������� | |
| B�� | ������ʵĴ������ܽ��ͷ�Ӧ��ܣ��Ӷ��ı䷴Ӧ���ʱ� | |
| C�� | ��pH��Ϊ2������ʹ���ֱ��к͵����ʵ�����NaOH�����Ĵ����������� | |
| D�� | ϡ�����м�������CH3COONa���壬����ĵ���̶ȼ�С��c��CH3COO-������ |
| A�� | �ýྻ�IJ�˿պȡ������Һ�ھƾ��ƻ��������գ�����ʻ�ɫ������Һ��һ��������K+ | |
| B�� | ����һ�����ʵ���Ũ�ȵ���Һʱ������ˮ��������ƿ�Ŀ̶��ߣ��ý�ͷ�ιܽ�����Һ���������� | |
| C�� | �ù�����NaOH��Һ����AlCl3��Һ��MgCl2��Һ | |
| D�� | ����ij��Һ���Ƿ���Fe2+ʱ�����ȼ�����������ˮ���ٵμ����軯����Һ������Һ��Ϊ��ɫ����˵����Һ��һ������Fe2+ |
| A�� | �������漰�ķ�Ӧ��Ϊ��������ԭ��Ӧ | |
| B�� | ��Ӧ�ڲ�������������ҪΪHCO3- | |
| C�� | ʵ��������ɷ�Ӧ��Ӧ���������н��� | |
| D�� | ��Ӧ�ܵ������Ǹ��¼��� |
| A�� | ��ҵ�Ͽ����Ȼ����Ʊ�����NH4Cl | |
| B�� | �ȼҵ�У����۵�����������NaOH | |
| C�� | �ڽӴ��ұ�������SO3��SO3���������ڱ�ˮ�����Ƴ�Ũ���� | |
| D�� | ��ҵ����ʯӢ��̫���ܵ�أ��ڸ����������ȷ������Ʊ��ֲ� |
| Ԫ�� | �й���Ϣ |
| X | Ԫ����Ҫ�������ϼ�Ϊ-2��-1�ۣ�ԭ�Ӱ뾶Ϊ0.074nm�� |
| Y | ��������������������������֮��Ϊ4�� |
| Z | ԭ�Ӱ뾶Ϊ0.102nm����������������������Ӳ�����2��������̬�⻯����Y���ʷ�����Ӧ���ɵ���ɫ���壮 |
| D | ����������Ӧ��ˮ����ܵ����������������������ȵ����������ӣ� |
| E | �����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
| F | Ԫ�����ڵ����������������������� |
��1��Z�����ڱ��е�λ�õ������ڵڢ�A�壬D������������Ӧˮ����ĵ���ʽΪ

��2��X������D���ʷ�Ӧ���ɵ�D2X2�ܺ�H2O��Ӧ��д�������ӷ���ʽΪ2Na2O2+2H2O�T4Na++4OH-+O2����
��3��EԪ����YԪ�ؿ��γ�EY2��EY3���ֻ��������۵⻯����Һ�μӼ���EY3��Ũ��Һ��ԭ��ɫ��Һ�ɱ�Ϊ��ɫ����ԭ����2Fe3++2I-�T2Fe2++I2�������ӷ���ʽ��ʾ����
��4��E�����ڷ���������ʴʱ��������Ӧ����ʽO2+2H2O+4e-�T4OH-
��5��F��һ�ָ��γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��Al3++3H2O�TAl��OH��3�����壩+3H+��
 ����B��E��Ϊͬ���칹�壬��A���ܵĽṹ�У�������
����B��E��Ϊͬ���칹�壬��A���ܵĽṹ�У�������| A�� | 8�� | B�� | 4�� | C�� | 2�� | D�� | 1�� |