��Ŀ����
�����Ѿ����Ƴ��Ա���Ϊȼ�ϵ�����ȼ�ϵ�أ������Ϊ����̼���Σ�����ܷ�Ӧ����ʽΪ��C3H8+5O2=3CO2+4H2O��
��1����֪��2C3H8��g��+7O2��g��=6CO��g��+8H2O��l��
C��s��+O2��g��=CO2��g��
2C��s��+O2��g��=2CO��g��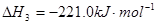
��ӦC3H8��g��+5O2��g��=3CO2��g��+4H2O��1���ġ�H___________________��.
��2���õ�ص�����ͨ��O2��CO2������ͨ����飬�������ĵ缫��ӦʽΪ_________________����ع���ʱCO32������_____________����
��3���øõ�ص��1L 1 mol��L��1��AgNO3��Һ���˵��ط�Ӧ�Ļ�ѧ����ʽΪ______________________�����õ������0.005molC3H8ʱ��������Һ��pHΪ__________����Һ����仯���Բ��ƣ�
��1����֪��2C3H8��g��+7O2��g��=6CO��g��+8H2O��l��

C��s��+O2��g��=CO2��g��

2C��s��+O2��g��=2CO��g��
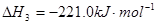
��ӦC3H8��g��+5O2��g��=3CO2��g��+4H2O��1���ġ�H___________________��.
��2���õ�ص�����ͨ��O2��CO2������ͨ����飬�������ĵ缫��ӦʽΪ_________________����ع���ʱCO32������_____________����
��3���øõ�ص��1L 1 mol��L��1��AgNO3��Һ���˵��ط�Ӧ�Ļ�ѧ����ʽΪ______________________�����õ������0.005molC3H8ʱ��������Һ��pHΪ__________����Һ����仯���Բ��ƣ�
��1����2221.5kJ��mol��1��2�֣���λ��λд�������֣�
��2��O2+2CO2+4e�� =2CO32����2�֣�����1�֣�
��3��4AgNO3+2H2O 4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣�
4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣�
��2��O2+2CO2+4e�� =2CO32����2�֣�����1�֣�
��3��4AgNO3+2H2O
 4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣�
4Ag+O2�� +4HNO3��2�֣���д��Ӧ��������ƽ�����֣� 1��2�֣������������1�����ݷ���ʽ�Ⱥ�˳����ֱ�Ϊ�١��ڡ��ۣ�
 ���õ�����ʽC3H8��g��+5O2��g��=3CO2��g��+4H2O��1������Ӧ��Ҳ������ʽ�����H=
���õ�����ʽC3H8��g��+5O2��g��=3CO2��g��+4H2O��1������Ӧ��Ҳ������ʽ�����H= =��2221.5kJ��mol��1��
=��2221.5kJ��mol��1����2��������������Ӧ��O2+2CO2+4e��=2CO32����ԭ��������������ƶ���
��3��������������Ƿ��������ͣ�4AgNO3+2H2O
 4Ag+O2�� +4HNO3�����õ������0.005molC3H8ʱ��ת�Ƶ�����0.1mol������0.1molH+��������Ũ��Ϊ0.1mol/L��pHΪ1��
4Ag+O2�� +4HNO3�����õ������0.005molC3H8ʱ��ת�Ƶ�����0.1mol������0.1molH+��������Ũ��Ϊ0.1mol/L��pHΪ1��
��ϰ��ϵ�д�
�����Ŀ

 FeO(s) +CO(g) ��H ="a" kJ/mol
FeO(s) +CO(g) ��H ="a" kJ/mol 
 H= ?241��8kJ/mol
H= ?241��8kJ/mol  2SO3(g)��
2SO3(g)�� 2NO(g)
2NO(g)  ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________. SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��



 CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1 CH3CH2OH(g)+3H2O(l) ��H= ��
CH3CH2OH(g)+3H2O(l) ��H= ��


 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 

 CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
 ��mol/L����2
��mol/L����2