��Ŀ����
15��ú��ʯ��������ʹ�õ���Ҫ��Դ��ͬʱҲ����Ҫ�Ļ���ԭ�ϣ���ͼ��ʾΪú��ʯ���ۺ����õIJ���;�����ش��������⣮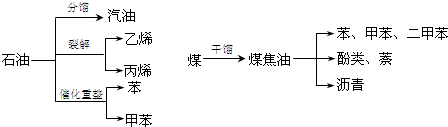
��1��ʯ�͵Ĵ�����ָʯ���е����������ڴ������¸ı���ӽṹ����������ɷ����������б仯�����������仯����A��
A��ʯ�͵ķ��� B��ʯ�͵��ѽ� C��ʯ�͵Ĵ����� D��ú�ĸ���
��2�����¸�������ֱ�����͡��ѻ����͡����༰�����еijɷ֣����������ṹд�����ƻ��������д���ṹ��ʽ��
��
 2��4-��������
2��4-����������
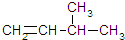 3-��-1-��ϩ
3-��-1-��ϩ��2-��-1-����

���ڼ�����
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
���ɱ�ϩ�ϳɾ۱�ϩ��֬��nCH2=CHCH3
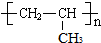
���ɼױ���ȡTNT���������ױ�����
 +3HO-NO2
+3HO-NO2
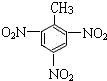 +3H2O
+3H2O�۹�ҵ������Ȳ��ȡ����ϩ��CH��CH+HCl
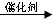 CH2=CHCl��
CH2=CHCl��
���� ��1��A��ʯ�͵ķ���������ʯ���и���ֵķе�IJ�ͬ�������ķ�����
B��ʯ�͵��ѽ����������͵ȳ������Ƶ���ϩ�ȶ������Ĺ��̣�
C��ʯ�͵Ĵ�����ָʯ���е����������ڴ������¸ı���ӽṹ����������ɷ������Ĺ��̣�
D��ú�ĸ����ǽ�ú����������ǿ����ú�ֽ�Ĺ��̣�
��2������������ʱ��ѡ���̼��Ϊ����������֧������һ�˸������ϵ�̼ԭ�ӱ�ţ�
��ϩ������ʱ��ѡ�������ŵ����̼��Ϊ��������������Ž���һ�˸�������̼ԭ�ӱ�ţ�
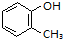
�۸������ƿ�֪����������4��̼ԭ�ӣ�-OH��1��̼ԭ���ϣ�����2��̼ԭ����
�������ƿ�֪�������ϵ�-OH�ͼ�����λ�Ĺ�ϵ���ݴ�д���ṹ��ʽ��
��3���ٱ�ϩ�����Ӿ۷�Ӧʱ���ǽ�̼̼˫���������Ӷ��γɸ߷��ӻ����
�ڼױ���Ũ������Ũ�����������������·���������Ӧ�����������ױ���
����Ȳ��HCl��������ȫ�ӳɿ����Ƶ�����ϩ��
��� �⣺��1��A��ʯ�͵ķ���������ʯ���и���ֵķе�IJ�ͬ�������ķ����������������̣���A��ȷ��
B��ʯ�͵��ѽ����������͵ȳ������Ƶ���ϩ�ȶ������Ĺ��̣������������ɣ��ǻ�ѧ�仯����B����
C��ʯ�͵Ĵ�����ָʯ���е����������ڴ������¸ı���ӽṹ����������ɷ������Ĺ��̣������������ɣ��ǻ�ѧ�仯����C����
D��ú�ĸ����ǽ�ú����������ǿ����ú�ֽ�Ĺ��̣��ǻ�ѧ�仯����D����
��ѡA��
��2������������ʱ��ѡ���̼��Ϊ��������������Ϊ6��̼ԭ�ӣ�����֧������һ�˸������ϵ�̼ԭ�ӱ�ţ�����2�ź�4��̼ԭ���ϸ���һ������������Ϊ��2��4-�������飬�ʴ�Ϊ��2��4-�������飻
��ϩ������ʱ��ѡ�������ŵ����̼��Ϊ����������������4��̼ԭ�ӣ���������Ž���һ�˸�������̼ԭ�ӱ�ţ���̼̼˫����1�ź�2��̼ԭ��֮�䣬��3��̼ԭ������4������������Ϊ��3-��-1-��ϩ��������Ϊ��3-��-1-��ϩ��
�۸������ƿ�֪����������4��̼ԭ�ӣ�-OH��1��̼ԭ���ϣ�����2��̼ԭ���ϣ��ʽṹ��ʽΪ��

�������ƿ�֪�������ϵ�-OH�ͼ�����λ�Ĺ�ϵ���ʽṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3���ٱ�ϩ�����Ӿ۷�Ӧʱ���ǽ�̼̼˫���������Ӷ��γɸ߷��ӻ������ѧ����ʽΪ��n CH2=CHCH3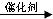
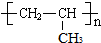 ���ʴ�Ϊ��n CH2=CHCH3
���ʴ�Ϊ��n CH2=CHCH3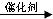
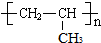
��
�ڼױ���Ũ������Ũ���������������ȵ������·���������Ӧ�����������ױ���ˮ���ʻ�ѧ����ʽΪ�� +3HO-NO2
+3HO-NO2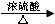
 +3H2O
+3H2O
�� +3HO-NO2
+3HO-NO2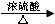
 +3H2O��
+3H2O��
����Ȳ��HCl��������ȫ�ӳɿ����Ƶ�����ϩ����ѧ����ʽΪ��CH��CH+HCl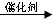 CH2=CHCl���ʴ�Ϊ��CH��CH+HCl
CH2=CHCl���ʴ�Ϊ��CH��CH+HCl CH2=CHCl��
CH2=CHCl��
���� ���⿼����ú��ʯ�͵��ۺ������Լ��л���������ͽṹ��ʽ����д��Ӧע����ճ������л���Ӧ�ķ�Ӧ�������ѶȲ���

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
| A�� | �ڣ�t1+10��minʱ�������������䣬����ѹǿ��ƽ�����淴Ӧ�����ƶ� | |
| B�� | ��t1+10��minʱ������������ѹǿ���䣬ͨ��ϡ�����壬ƽ�������ƶ� | |
| C�� | T��ʱ������ͬ�����У�����0.3 mol•L-1 A��0.1 mol•L-1 B��0.4 mol•L-1C��Ӧ���ﵽƽ���C��Ũ����Ϊ0.4 mol•L-1 | |
| D�� | �÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���A��ת�������� |
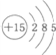 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ��ԭ��λ�ڵ������ڣ���A�� | B�� | ��ԭ��λ�ڵ������ڣ���B�� | ||
| C�� | ��ԭ��λ�ڵ������ڣ���B�� | D�� | ��ԭ��λ�ڵ������ڣ���A�� |
| �� | �� | �� | |
| A | Na | H2O | Cl2 |
| B | Al | Fe2O3 | ϡH2SO4 |
| C | CH3CH2OH | NaOH��Һ | CH3COOH |
| D | Ba��OH��2��Һ | CO2 | Na2CO3��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
��K+��NH4+��F-����K+��Ba2+��Cl-����K+��Na+��I-����K+��Na+��ClO-��
| A�� | �٢ڢۢ� | B�� | ֻ�Тڢۢ� | C�� | ֻ�Т٢ۢ� | D�� | ֻ�Тڢ� |
 ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף�
ij�¶�ʱ����һ��2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ�����ݣ�����д���пհף� ��ͼ������������CnH m������̼ԭ����n��Cԭ�ӣ��뺬̼��C%�Ĺ�ϵ��
��ͼ������������CnH m������̼ԭ����n��Cԭ�ӣ��뺬̼��C%�Ĺ�ϵ��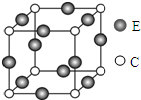 ��֪A��B��C��D��E����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ���ԭ��������������Ԫ��A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ���е���ռ��������������ͬ��ԭ�ӹ�����������ֹ���еĵ�������ͬ��Dλ�ڵ�2���ڣ���ԭ�Ӻ���ɶԵ�������δ�ɶԵ�������3����Eλ��ds������ԭ�ӵ�������������A����ͬ��
��֪A��B��C��D��E����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ���ԭ��������������Ԫ��A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ���е���ռ��������������ͬ��ԭ�ӹ�����������ֹ���еĵ�������ͬ��Dλ�ڵ�2���ڣ���ԭ�Ӻ���ɶԵ�������δ�ɶԵ�������3����Eλ��ds������ԭ�ӵ�������������A����ͬ��