��Ŀ����
7������˵�����ʾ����ȷ���ǣ�������| A�� | ������������Ӧ���ɵ�����ˮ������Һ̬ˮ��ǰ�߷ų������� | |
| B�� | ��Ҫ���ȵķ�Ӧ˵���������ȷ�Ӧ | |
| C�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O����H=-57.3kJ/mol��������0.5molH2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ | |
| D�� | 1molS��ȫȼ�շ���297.3kJ�����Ȼ�ѧ����ʽ��S+O2�TSO2����H=-297.3 kJ/mol |
���� A��Һ̬ˮ��Ϊ��̬ˮ�Ĺ��������ȵģ�
B���еķ��ȷ�ӦҲ����Ҫ���ȵģ�
C��Ũ����ϡ�ͷ��ȣ�
D�������Ȼ�ѧ����ʽ����д�������ش�
��� �⣺A����ΪҺ̬ˮ��Ϊ��̬ˮ�Ĺ��������ȵģ�����������������Ӧ���ɵ�����ˮ������Һ̬ˮ�����߷ų������࣬��A����
B�����ȷ�Ӧ��Ҫ�����²��ܷ������������Ƿ��ȷ�Ӧ����B����
C��Ũ����ϡ�ͷ��ȣ�����0.5molH2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ����C��ȷ��
D��1molS��ȫȼ�շ���297.3 kJ�����Ȼ�ѧ����ʽΪ��S��s��+O2��g���TSO2��g������H=-297.3 kJ/mol����D����
��ѡC��
���� ���⿼��ѧ���Ȼ�ѧ����ʽ����д�������Լ��к��ȵĸ����֪ʶ�������ۺ�֪ʶ�Ŀ��飬�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
18�����л������������Ӱ뾶�����ǣ�������
| A�� | LiI | B�� | NaBr | C�� | KCl | D�� | CsF |
12������˵������ȷ���ǣ�������
| A�� | 1mol�� | B�� | 1molH2SO4 | C�� | 1mol�� | D�� | 1mol��� |
19��������ʵ�뽺�������ص��ǣ�������
| A�� | �ڶ����������±������ | |
| B�� | �ں����뺣�����γ�ɳ�� | |
| C�� | һ��ƽ�й������䵰������Һʱ���Ӳ�����Կ���һ��������ͨ· | |
| D�� | FeCl3��Һ�е���NaOH��Һ���ֺ��ɫ���� |
17����֪����Ԫ�ص�ԭ�Ӱ뾶�������ϱ����ݷ�������ԭ�Ӱ뾶�����ǣ�������
| ԭ�� | N | S | Si |
| �뾶r/10-10m | 0.75 | 1.02 | 1.17 |
| A�� | 1.10��10-10m | B�� | 0.70��10-10m | C�� | 1.20��10-10m | D�� | 0.80��10-10m |
 �����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�
�����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã� ��
�� ����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ
����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ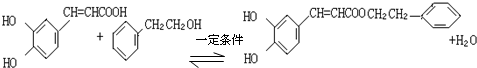 ��
�� ��һ���º����ܱ������У�ij��ѧ��Ӧ2A��g��?B��g��+C��g�������ֲ�ͬ�����½��У�����ʵ�����800�棬ʵ�����820�棬B��C����ʼŨ�ȶ�Ϊ0����Ӧ��A��Ũ�ȣ�mol•L-1����ʱ�䣨min���仯��ͼ��ʾ��
��һ���º����ܱ������У�ij��ѧ��Ӧ2A��g��?B��g��+C��g�������ֲ�ͬ�����½��У�����ʵ�����800�棬ʵ�����820�棬B��C����ʼŨ�ȶ�Ϊ0����Ӧ��A��Ũ�ȣ�mol•L-1����ʱ�䣨min���仯��ͼ��ʾ��