��Ŀ����
15�� �����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�
�����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã��ٿ�������NaHCO3��Һ��Ӧ�ķ���ʽ
 ��
���ڷ佺����Ҫ���Գɷ�ΪCPAE������ʽΪC17H16O4����������һ�������¿�ˮ�����ɿ������һ�ִ����ô�Ϊ���㴼�ҷ��ӽṹ�������˷��㴼�Ľṹ��ʽΪ
 ����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ
����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ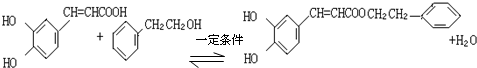 ��
��
���� �ٺ����Ȼ�������̼�����Ʒ�Ӧ���ɶ�����̼���壻
�ڷ���ʽΪC17H16O4����������һ�������¿�ˮ�����ɿ������һ�ִ���˵��CPAE�����������ô�Ϊ���㴼�ҷ��ӽṹ�������ɷ���ʽ��֪�ô�Ϊ ���Դ˽��
���Դ˽��
��� �⣺�ٺ����Ȼ�������̼�����Ʒ�Ӧ���ɶ�����̼���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
�ڷ���ʽΪC17H16O4����������һ�������¿�ˮ�����ɿ������һ�ִ���˵��CPAE�����������ô�Ϊ���㴼�ҷ��ӽṹ�������ɷ���ʽ��֪�ô�Ϊ ����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ
����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
���� ���⿼���л���Ľṹ�����ʣ���Ŀ�ѶȲ���ע���л���ĺ��еĹ����ŵ����ʣ���Ϊ������Ĺؼ�����Ϥ���ӡ�ϩ������������ʼ��ɽ��

��ϰ��ϵ�д�
�����Ŀ
5����X��Һ�л����μ�Y��Һ�����ɳ�����������ͼ��ʾ������ͼ���һ���ǣ�������


| A�� | X��AlCl3��Mg��NO3��2��HNO3��Y��NaOH | |
| B�� | X��Na2CO3��NH4HCO3��Na2SO4��Y��Ba��OH��2 | |
| C�� | X��NH4NO3��Al��NO3��3��Fe��NO3��3��HCl��Y��NaOH | |
| D�� | X��NaAlO2����ˮ��NaOH��Y��H2SO4 |
6���ȿ�������ȥ�����е���ϩ���ֿ����������������ϩ�ķ����ǣ�������
| A�� | ������ͨ������Һ���� | B�� | �ڵ��ܿڴ���ȼ | ||
| C�� | һ����������H2�����ӳɷ�Ӧ | D�� | ������ͨ��������ˮ�� |
3�����ܱ������У����з�ӦX��g��+3Y��g��?2Z��g�����ﵽƽ��������������䣬ֻ����X������������������ȷ���ǣ�������
| A�� | ����Ӧ���������淴Ӧ���ʼ�С | B�� | X��ת���ʱ�� | ||
| C�� | Y��ת���ʱ�� | D�� | ��ƽ��ʱX�����������С |
20�������г��˵�ⲻͬ���ʵĵ缫��Ӧʽ�����д�����ǣ�������
| A�� | ��ⱥ��ʳ��ˮ�� ������Na++e-�TNa | |
| B�� | ���ͭʱ���������ҺΪCuSO4��Һ���� ������Cu-2e-�TCu2+ | |
| C�� | �������NaCl�� ������Na++e-�TNa | |
| D�� | ���NaOH��Һ�� ������4OH--4e-�T2H2O+O2�� |
7������˵�����ʾ����ȷ���ǣ�������
| A�� | ������������Ӧ���ɵ�����ˮ������Һ̬ˮ��ǰ�߷ų������� | |
| B�� | ��Ҫ���ȵķ�Ӧ˵���������ȷ�Ӧ | |
| C�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O����H=-57.3kJ/mol��������0.5molH2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ | |
| D�� | 1molS��ȫȼ�շ���297.3kJ�����Ȼ�ѧ����ʽ��S+O2�TSO2����H=-297.3 kJ/mol |
5����100mL���������Ļ����Һ�У�c��HNO3��=0.2mol/L��c��H2SO4��=0.4mol/L�������м���2.56gͭ�ۣ��ȣ���ַ�Ӧ����Һ��c��Cu2+��Ϊ����Ӧǰ����Һ������ֲ��䣩��������
| A�� | 0.40mol/L | B�� | 0.30mol/L | C�� | 0.075mol/L | D�� | ������ |
