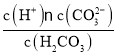��Ŀ����
����Ŀ�����ǵ������ڵ���A��Ԫ�أ�������Ҫ�İ뵼����ϣ��������쾧��ܼ����ֵ���װ�á���ͼΪ����п��(��GeO2��ZnS������Fe2O3)Ϊԭ�������ߴ�����Ĺ����������£�

��֪��GeO2������ǿ����Һ�����������Ρ�GeCl4���۵�Ϊ��49.5�����е�Ϊ84������ˮ�л����ϡ��Һ����ˮ�⡣
�ش��������⣺
(1)������������Ч�ʵĴ�ʩ��______________________(дһ��)��NaOH��Һ���ʱ������Ӧ�����ӷ���ʽΪ______________________��
(2)����a��������___________��GeCl4�ĵ���ʽΪ_______________��
(3)����1�г�����ZnS�⣬����������___________������2�г����� CaGeO3�⣬����������___________��
(4)�������м���Ũ���ᣬ������Ӧ�Ļ�ѧ����ʽΪ______________________������ϡ�����ԭ����______________________��
(5)д��һ��֤����������Ӧ��ȫ�IJ���������______________________��
���𰸡�����ʯ����(����Ȼ��ֽ���) GeO2+ 2OH- =GeO32-+H2O ����  Fe2O3 Ca(OH)2 CaGeO3+6HCl(Ũ)=CaCl2+ CeCl4+3H2O GeCl4��ϡ��������ˮ�� ����Ӧ���ɵ�����ͨ��װ����ˮ����ͭ��U�ιܣ�����δ����
Fe2O3 Ca(OH)2 CaGeO3+6HCl(Ũ)=CaCl2+ CeCl4+3H2O GeCl4��ϡ��������ˮ�� ����Ӧ���ɵ�����ͨ��װ����ˮ����ͭ��U�ιܣ�����δ����
��������
�����п��(��Ҫ�ɷ�GeO2��ZnS������Fe2O3)������Һ�к��������Σ������Ȼ�����Һ��Ӧ�γ�CaGeO3���������˺�õ�CaGeO3����CaGeO3��Ũ�����ܽ�����GeCl4��GeCl4��Ũ�������ܽ�ȵͣ����˺�õ�GeCl4��GeCl4�ڴ�ˮ��ˮ������GeO2nH2O��GeO2nH2O��ˮ��õ�GeO2�����������Ȼ�ԭGeO2�õ��ߴ���Ge���ݴ˷������
(1)�����������Ч�ʵĴ�ʩ�н���ʯ�������Ȼ��ֽ��裬NaOH��Һ���ʱ����GeO2�ܽ����������Σ���Ӧ�����ӷ���ʽΪGeO2+ 2OH- =GeO32-+H2O���ʴ�Ϊ������ʯ����(����Ȼ��ֽ���)��GeO2+ 2OH- =GeO32-+H2O��
(2)����aΪ������Һ�������Ϊ���˲�����GeCl4���۵�Ϊ��49.5�棬�е�Ϊ84�棬Ϊ���Ӿ��壬���й��ۼ�������ʽΪ ���ʴ�Ϊ�����ˣ�
���ʴ�Ϊ�����ˣ� ��
��
(3)�������������������ƣ�����1�г�����ZnS�⣬����������Fe2O3��������������ˮ���Ȼ����ܹ����������Ʒ�Ӧ�����������Ƴ������������2�г����� CaGeO3�⣬����������Ca(OH)2���ʴ�Ϊ��Fe2O3��Ca(OH)2��
(4)��������ͼ��������м���Ũ���ᣬ��Ӧ����CeCl4����Ӧ�Ļ�ѧ����ʽΪCaGeO3+6HCl(Ũ)=CaCl2+ CeCl4+3H2O��GeCl4��ϡ��������ˮ�⣬��˲���ϡ�����ܽ⣬�ʴ�Ϊ��CaGeO3+6HCl(Ũ)=CaCl2+ CeCl4+3H2O��GeCl4��ϡ��������ˮ�⣻
(5)����������������Ȼ�ԭGeO2�õ��ߴ���Ge������Ӧ��ȫ���ٲ���ˮ���������Խ���Ӧ���ɵ�����ͨ��װ����ˮ����ͭ��U�ιܣ�����δ����������֤����Ӧ�Ѿ���ȫ���ʴ�Ϊ������Ӧ���ɵ�����ͨ��װ����ˮ����ͭ��U�ιܣ�����δ������

 �Ķ��쳵ϵ�д�
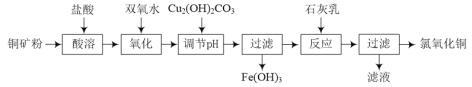
�Ķ��쳵ϵ�д�����Ŀ����ҵ���Ը�����(��Ҫ�ɷ���FeO��Cr2O3��������MgCO3��Al2O3��SiO2��)Ϊԭ����ȡ������(Na2CrO4)���壬�乤���������£�

��֪������3��Cr��������Һ�������ȶ�����pH>9ʱ��CrO![]() ��ʽ�������ױ�������
��ʽ�������ױ�������
�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3�� | Fe2�� | Mg2�� | Al3�� | Cr3�� |
��ʼ����ʱ��pH | 1��9 | 7��0 | 9��1 | �� | �� |
������ȫʱ��pH | 3��2 | 9��0 | 11��1 | 4��7(>10�ܽ�) | 5��6(>9�ܽ�) |
(1)���������ʵĴ�ʩ��__________________________________(������)��
(2)����1����Ҫ�ɷ���_____������2����Ҫ�ɷ���_____������3����Ҫ�ɷ���_____��
(3)����������ʹ����H2O2����������H2O2�ĵ���ʽΪ___________����һ������ʱ��Ӧ�����ӷ���ʽΪ_____________________���ڶ�������ʱĿ����____________________��
(4)����ͼ���� ���ڵIJ�����________________��ϴ�ӡ����
(5)��ȥ���Է�ˮ�к��е�Cr2O72-����ʹ��FeSO4����÷�Ӧ�����Һ�к�Cr3����Fe2����Fe3����H���������ӡ��÷�Ӧ�����ӷ���ʽΪ_________________________________��