��Ŀ����
��18.4mol/L��Ũ����ϡ�ͳ�0.92mol/L��ϡ����100ml,�ش��������⣺
��1��
| ӦȡŨ��������/ml | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| | | |
��2�����Ʋ����ɷֽ�����¼�����
A��������ƿ��ע����������ˮ���������ƿ�Ƿ�©ˮ
B������������ˮϴ���ձ�������Һע������ƿ�����ظ���������
C��������ȴ������ע������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ�ȣ�װƿ
G���ý�ͷ�ιܼ���������ˮ��ʹ��Һ����ǡ����̶�����
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
��ȷ�IJ���˳���ǣ�A____________________________F
��3�����в��������ʹ��Һ���ʵ���Ũ��ƫ�͵���
A��û�н�ϴ��Һת�Ƶ�����ƿ��
B������ƿϴ����δ�����ﴦ��
C��ת�ƹ���������������Һ����
D��ҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶���
��1��5.0��2�֣���100��2�֣�����Ͳ���ձ�������������ͷ�ιܣ�2�֣�
��2��DECBHG��3�֣� ��3��AC��3�֣�
���������������1����18.4mol/L��Ũ����ϡ�ͳ�0.92mol/L��ϡ����100ml,�����ϡ���������ʲ����֪����ҪŨ����������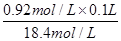 ��0.005L��5.0ml������ӦȡŨ����������5.0ml������100ml���ᣬ����Ҫ100ml����ƿ�������Ҫ��Ͳ��ȡŨ���ᣬϡ��Ũ�������ձ���ϡ�ͺ�ת�ƻ���Ҫ������������ʱ����Ҫ��ͷ�ιܡ�
��0.005L��5.0ml������ӦȡŨ����������5.0ml������100ml���ᣬ����Ҫ100ml����ƿ�������Ҫ��Ͳ��ȡŨ���ᣬϡ��Ũ�������ձ���ϡ�ͺ�ת�ƻ���Ҫ������������ʱ����Ҫ��ͷ�ιܡ�
��2������ƿ��ʹ��ǰ��Ҫ��©������IJ��������м��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ��������������˳����A DECBHGF��
��3������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�����������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��A��û�н�ϴ��Һת�Ƶ�����ƿ�У������ʼ��٣�����Ũ��ƫ�ͣ�B������ƿϴ����δ�����ﴦ������Ӱ��ʵ��������Ũ�Ȳ��䣻C��ת�ƹ���������������Һ�����������ʼ��٣�����Ũ��ƫ�ͣ�D��ҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶��ߣ�Ũ�Ȳ��䣬��˴�ѡAC��
���㣺����һ�����ʵ���Ũ�ȵ����ơ�����ѡ���Լ���������

�����Ͻ���һ�������½ṹ���ϡ�
��1��ͼ9��ʾ����ʱ��ͬ��ݵ�Fe3Al��65%ŨHNO3 �еĸ�ʴ�������ͼ9�ɿ��������� ������ţ�Ԫ�صĺϽ���ʴ����
��2�����������£�Al��Fe2O3��һ��������Ϸ�Ӧ����
|
��С��ͬѧ������·�����ȷ��ȡ1.46g�úϽ��ĩ���ټ�������NaOH��Һ��Si+2NaOH+H2O��Na2SiO3+2H2��������ַ�Ӧ����ˣ�ͨ���ⶨʣ������������ռ��������������ɼ�����˺Ͻ����ɡ�д��Al��NaOH��Һ��Ӧ�����ӷ���ʽ ��
��С��ͬѧ��Ϊ�÷������Բⶨ��״����������������������㡣����������˵ڶ��ַ�����ȷ��ȡ1.46g�úϽ��ĩ���������������Һ��ַ�Ӧ����ˣ��ⶨʣ���������0.07g������Һ�еμ�����ŨNaOH��Һ����ֽ��衢���ˡ�ϴ�ӵù��塣�ٽ����ù����ּ��ȡ����յú���ɫ��ĩ1.60g����ͨ������ȷ���˺Ͻ����ɡ�
�Ȼ�����Һ�������������ж��й㷺����;��������1 L 0.2 mol��L-1 NaCl��Һ����ش����⡣
| ʵ�鲽�� | �й����� |
| ��1�����㲢���� | ��������ƽ����NaCl���������Ϊ g |
| ��2���ܽ� | Ϊ�����ܽ⣬�ɽ��еIJ����� |
��3��ת�� | ָ��ʾ��ͼ�е��������� ����1�� ����2�� |
| ��4��ϴ�� | ����������ˮϴ�� 2~3�Σ�����ϴ��Һת�Ƶ�����ƿ�� |
| ��5������ | ����������ƿ�̶��ߣ����ݺ�����������Һ�����ʵ���Ũ�� ���ƫ�ߡ���ƫ�͡�����Ӱ�족�� |
��18.4mol��L-1��ŨH2SO4����100mLŨ��Ϊ1mol��L-1��H2SO4��Һ��������ɷ�Ϊ���¸�����
| A������Ͳȡ��mLŨH2SO4����ע��װ��Լ50mL����ˮ���ձ���,���ò��������Ͻ��� |
| B����Լ30mL����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴҺ������100mL����ƿ�� |
| C����ϡ�ͺ�H2SO4��ҺС�ĵ���100mL����ƿ�� |
| D�����100mL����ƿ�ڲ��Ƿ�ᷢ����© |
��F���ǽ�ƿ���������ߵ���ҡ����Һ
��G������������������ƿ����ε�������ˮ����Һ����͵�ǡ�úͻ��ο̶�������
�ݴ���д����1�����������Ŀհ״���
��2����ȷ�IJ���˳���ǣ���ĸ��д������������
��3������A������Ӧѡ��������������10mL��Ͳ����50mL��Ͳ��500mL��Ͳ����1000mL��Ͳ�еģ�����ţ�������������
����Ԫ���У����ڷǽ���Ԫ�ص���
| A��Ca | B��C | C��Na | D��Al |
����ѧ����������Ĺؼ���������˵������ȷ���� (����)
| A��PM2.5��ָ������ֱ����2.5 ��m�Ĺ��������Һ�ε��ܳ� |
| B�����ݷ�ɢ�����ӵ�ֱ����С����ɢϵ�ɷ�Ϊ��Һ����Һ�ͽ��壬��Һ�ķ�ɢ�����Ӵ�С������Һ�뽺��֮�� |
| C����ѧ�ҷ���һ����ϸ����DNA�������飨As��Ԫ�أ���AsԪ�����п���ȡ������ͨDNA���е�PԪ�� |
D��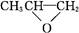 ��CO2��Ӧ���ɿɽ���ۺ��� ��CO2��Ӧ���ɿɽ���ۺ���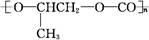 ���÷�Ӧ������ɫ��ѧ��ԭ�� ���÷�Ӧ������ɫ��ѧ��ԭ�� |
���෨��һ����֮��Ч�������еĿ�ѧ��������������ʶ����ʱ����ȡ���ַ���������и�������в���������
| ѡ�� | ��ijһ����������һ�� | ���� |
| A | FeSO4 ��NO2 ��MnO2��NaClO | H2SO3 |
| B | CH3COOH��HClO��H2S��HOOC-COOH | HF |
| C | �Ӿ۷�Ӧ�����ӷ�Ӧ�����ȷ�Ӧ��������ԭ��Ӧ | ��ɫ��Ӧ |
| D | (NH4)2SO4��NH4Cl��NH4NO3��NH3��H2O | NH4HCO3 |