��Ŀ����
��þ�����������Σ��������Ʊ����ᣨH3BO3����MgO���������£���þ�����(NH4)2SO4��Һ��Ϻ���ȣ���Ӧ����H3BO3�����MgSO4��Һ��ͬʱ�ų�NH3������MgSO4��Һ��ͨ��NH3��CO2���õ�MgCO3��������Һ��������ϴ�ӡ����պ��MgO����Һ��ѭ��ʹ�á��ش��������⣺
��1������������ƣ������νṹҲ�Ƚϸ��ӣ���Ӳ���ʯ��ѧʽΪCa2B6O11��5H2O�������дΪ���������ʽ ��
��2�������Ʊ������У��������ϴ���Ƿ���ȫ�ķ����� ��
��3��д��MgSO4��Һ��ͨ��NH3��CO2��Ӧ�Ļ�ѧ����ʽ ��
��4����ȷ��ȡ1.68 g��þ����ȫ��Ӧ���H3BO3����1.24 g��MgO 0.8 g������������ε���ɡ���д��������̣�
��1��2CaO��3B2O3��5H2O��2�֣�
��2��ȡ�������һ��ϴ����Һ������1��2��Ba(NO3)2��Һ���������ְ�ɫ���ǣ���ʾ��ϴ����ȫ����2�֣�
��3��MgSO4+CO2+2NH3+H2O=MgCO3+(NH4)2SO4��2�֣�
��4��n (B2O3)=0.01mol��1�֣�
n (MgO)="0.02mol" ��1�֣�
n (H2O) =" (1.68" g -0.7 g -0.8 g) /18 g��mol-1=" 0.01mol" ��2�֣�
�������Ϊ��Mg2B2O5��H2O��2MgO��B2O3��H2O����2�֣�
��˵�������������ⷨ��ɣ�B��Mg���ʵ�����1�֣�����ˮ�����ʵ�����1�֣����2�֣�
���������������1�����ջ�ѧʽ��Ԫ�ص�˳������д�����������ʽ��ע��Ԫ�صĻ��ϼۺ�ԭ����Ŀ�Ĺ�ϵ����2���������ϴ���Ƿ���ȫ�ķ���Ҫע��ȡ���һ�ε�ϴ��Һ�������Լ���ѡ������������ӵ������ԡ��������Լ�������Ҫ˵��������۵����ݣ���4������ĵڣ�1������������������ʽ���ʸ���ļ���Ӧ����þ������Ӧ�������������ȷ���Ƿ���ˮ��
n (B2O3)=0.01mol
n (MgO)="0.02mol"
n (H2O) =" (1.68" g -0.7 g -0.8 g) /18 g��mol-1=" 0.01mol"
���������Mg2B2O5��H2O��2MgO��B2O3��H2O��
���㣺�������ʵ��Ʊ��з�Ӧ����ʽ����д��ʵ���������ѧʽ��ȷ�����й����⡣

��18.4mol/L��Ũ����ϡ�ͳ�0.92mol/L��ϡ����100ml,�ش��������⣺
��1��
| ӦȡŨ��������/ml | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| | | |
��2�����Ʋ����ɷֽ�����¼�����
A��������ƿ��ע����������ˮ���������ƿ�Ƿ�©ˮ
B������������ˮϴ���ձ�������Һע������ƿ�����ظ���������
C��������ȴ������ע������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ�ȣ�װƿ
G���ý�ͷ�ιܼ���������ˮ��ʹ��Һ����ǡ����̶�����
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
��ȷ�IJ���˳���ǣ�A____________________________F
��3�����в��������ʹ��Һ���ʵ���Ũ��ƫ�͵���
A��û�н�ϴ��Һת�Ƶ�����ƿ��
B������ƿϴ����δ�����ﴦ��
C��ת�ƹ���������������Һ����
D��ҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶���
�����Ǵ�������ǣ��� ��
| A�����ȼ� | B���ƾ� | C��ֲ���� | D������ |
���dz��á����˿������Ρ�������ijЩ�˳���֮Σ����Ϊ����ʵ�ӻ�ѧ�ĽǶ���˵�������˿������Ρ������������ף���������˵�����Ǻ��˶��Ǿ��ˡ���ô�����������Ļ�ѧԭ����
| A������ĵ�Ӿ | B��ѪҺ��������ԭ��Ӧ |
| C��ѪҺ�з������ֽⷴӦ | D������ľ۳� |
 ��
��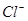 �����ʵ���Ũ�ȣ��ٶ��ڻ��Һ�м�������������ĩ�����δ�ı䣩��
�����ʵ���Ũ�ȣ��ٶ��ڻ��Һ�м�������������ĩ�����δ�ı䣩��