��Ŀ����
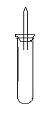
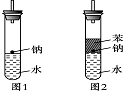
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ���������ɽ����Ʊ�H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��2Al+2NaOH+2H2O=2NaAlO2+3H2������Ϊ��ȼ����������Ӧ���ɵ�H2���������������ͼ��ʾ��װ�á�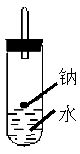
��ش��������⣺
��1��Ҫȡ�ý���ú���еĽ���Na����ʵ�飬����ȷ�IJ���������___________________��
��2��д��Na��H2O��Ӧ�����ӷ���ʽ��_____________________________��
��3���ڵ�ȼH2֮ǰ�����Ƚ���________________��������_________________________________��
��4����ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ���٢�ʵ�����˳ɹ�����ȴʧ���ˡ��������������������Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����ǣ�����������Na������������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����__________________��
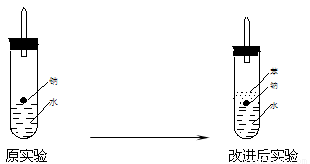
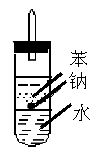
��5����ʵ��С����ĵ�Na������һ�ֲ���Na��Ӧ��Һ̬�л����ˮ���ܶȷֱ�Ϊ0.97g?cm-3��0.88g?cm-3��1.00g?cm-3�����ݴ˶�ʵ�����������ͼ��ʾ�ĸĽ����ڸĽ����ʵ���У�H2���������ʼ�����ԭ����_____________________________________________________��
��1��������ȡ���ƿ飬����ֽ�����ƿ�����ú�ͣ��ڲ���Ƭ����С����ȥ�Ʊ���������㣬������һС���Ʊ��ã����µ���ȫ���Ż�ԭ�Լ�ƿ
��2��2Na+2H2O��2Na++2OH-+H2��
��3���鴿 �������ſ������ռ�һС�Թ���������Ĵָ��ס�Թܿڣ��ƽ�������ɿ�Ĵָ������������ġ��ˡ����������H2����
��4���϶��Na��ˮ��Ӧ�ų��������ȣ�ʹ�Թ��ڵ�H2��O2��ϵ�ȼ����ը
��5��Na���ܶȱ�ˮ��С�����ȱ��Ĵ��佫���ڱ���ˮ�Ľ��紦��Na��H2O��Ӧ������H2ʹNa��������ˮ�棬��Ӧֹͣ����Na�����H2�ݳ�ʱ��Na�ֻ��䵽���紦����ˮ��Ӧ����˷������Ϳɼ���Na��H2O��Ӧ������
�����������

��ϰ��ϵ�д�
�����Ŀ
 ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��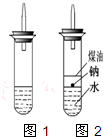 ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��
ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��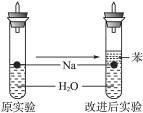
 (3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��