��Ŀ����
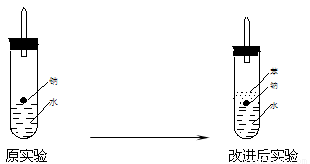
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о�,�ܽ��������������H2�ķ�Ӧ:��Zn+����;��Na+ˮ;��Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2,�������������ͼ��ʾ��װ��ͼ:
![]()
]��ش���������:
(1)д��Al��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________________��
(2)�ڵ�ȼH2֮ǰ�����Ƚ���____________________________________________��
(3)ʵ��С���ڵ�ȼ������װ���Ƶõ�H2ʱ,�٢�ʵ���óɹ�,��ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫��,Na��������̫�١����������������Ƶ�����,�ɽ�ʦ˵̫Σ��,����Ϊ����Σ�յ�ԭ����___________________________��
(4)ʵ��С������ơ���(һ�ֲ�����ˮ��Һ̬�л���)��ˮ���ܶȷֱ�Ϊ0.97 g��mL-1��0.88 g��mL-1��1.00 g��mL-1,���ݴ˶�ʵ������˸Ľ����ڸĽ����ʵ����H2����������______________________��(��������ӿ족)
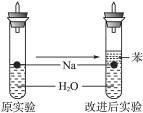
(5)2.3 g��Ͷ��20 mLˮ����ȫ��Ӧ�ų��������ڱ�״���µ������_____________,������Һ�����ʵ���Ũ����______________________��(������Һ����ı仯)
(1)2Al+2H2O+2NaOH====2NaAlO2+3H2��(2)�鴿
(3)�϶������ˮ��Ӧ�ų���������,ʹ�Թ���H2��O2�Ļ������ȼ����ը
(4)����(5)1.12 L 5 mol��L-1
����:
��ȼ�������ڵ�ȼǰ�����鴿,���ⱬը�����Թ��в�Ҫ�������Na,����϶������ˮ��Ӧ�ų���������,ʹ�Թ���H2��O2�Ļ������ȼ����ը;�ƿ����ḽ�ű�,���ԸĽ����ʵ����H2���������ʼ���;2Na+2H2O====2NaOH+H2��,0.1 mol Na��Ӧ�ų�����Ϊ1.12 L;������Һ�����ʵ���Ũ��=![]() ��
��

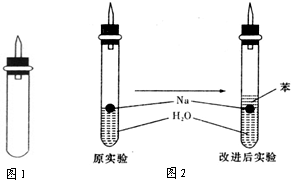 ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��
ijʵ��С�����ѧ�α��п����������ķ�Ӧ�������о����ܽ��������������H2�ķ�Ӧ����Zn+�����Na+ˮ����Al+NaOH��Һ��Ϊ��ȼ����������Ӧ���ɵ�H2�������������ͼ��ʾ��װ��ͼ��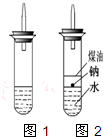 ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��
ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��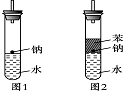 (3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��
(3)ʵ��С���ڵ�ȼ��ͼ1װ���Ƶõ�H2ʱ���٢�ʵ���óɹ�����ȴʧ���ˡ����Ƿ�����Ϊʧ�ܵ�ԭ����Na��H2O�ķ�Ӧ����̫�죬Na������̫�١����������������Ƶ�����������ʦ˵̫Σ�գ�����Ϊ����Σ�յ�ԭ����________________________________________________________________________��