��Ŀ����
����Ŀ�����������ʣ��������������塡��KHSO4����HNO3 ��ϡ���� �ݶ�����̼���塡��ͭ����̼���Ʒ�ĩ�������Ǿ��塡�������Ȼ��ơ���CuSO4��5H2O���塣���������գ�
(1)����״̬�¿ɵ������____________________________________��
(2)���ڵ���ʵ���__________________________________________��
(3)���ڷǵ���ʵ���____________________________________��
(4)����ˮ��Һ�еĵ��뷽��ʽΪ_____________________�����������Һ�з�Ӧʹ��Һ�����Ե����ӷ���ʽΪ______________________________________________��
(5)������Է������·�Ӧ��Cu��4HNO3(Ũ) ��Cu(NO3)2��2NO2����2H2O���ش��������⣺
I����ԭ������________��
II������2 mol HNO3�μӷ�Ӧʱ�������������ʵ�����Ϊ________g��
���õ����ŷ������Ӧ�е���ת�Ƶķ������Ŀ________________________________��
���𰸡��ܢޢ� �٢ڢۢߢ�� �ݢ� KHSO4��K+ + H+ +SO42- Ba2++2OH-+2H++SO42-��BaSO4��+2H2O NO2 32 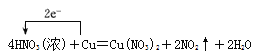
��������
��1����������ʰ������������ʡ�ʯī�����ڵļ���Ρ����ˮ��Һ��ˮ���������״̬�µ�����Ǣܢޢ
��2������ʰ����ᡢ��������Ρ������Ľ��������ˮ���������ڵ���ʵ��Ǣ٢ڢۢߢ�⣻
��3���ǵ����Ϊ������ʵĻ�������������ڷǵ���ʵ��Ǣݢࣻ
��4��KHSO4��ˮ�е��뷽��ʽΪKHSO4=K����H����SO42����Ba(OH)2��KHSO4��Ӧ����Һ�����ԣ��������ʵ���֮��Ϊ1��2��������ӷ���ʽΪBa2����2OH����2H����SO42��=BaSO4����2H2O��
��5��I.��ԭ����������������ԭ�IJ�����ݷ�Ӧ����ʽ��HNO3��N�Ļ��ϼ۽��ͣ�HNO3Ϊ����������NO2Ϊ��ԭ���
II.���ݷ�Ӧ����ʽ��2molHNO3�μӷ�Ӧʱ����������Cu������Ϊ2��64/4g=32g��
III.�����Ŵӻ��ϼ����ߵ�Ԫ�س�����ָ�ϼ۽��͵�Ԫ�أ�1molCu���뷴Ӧ��ת�Ƶ������ʵ���Ϊ2mol������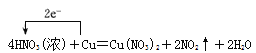 ��
��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ������β����ͨ������NxOy��CO�ȴ�����Ⱦ���ѧ��Ѱ�Ҹ�Ч����ʵ��������β����ת��������������β���Դ�������Ⱦ��
��1����֪����CO��ȼ������H1=-283kJ��mol-1 ��N2��g��+O2��g�� =2NO��g�� ��H2 =+183kJ��mol-1
�ɴ�д��NO��CO��Ӧ���Ȼ�ѧ��Ӧ����ʽ________________________________��
��2��һ���¶��£���2L�ܱ������г���4.0mo1NO2��4.0molCO���ڴ��������·�����Ӧ 4CO��g��+2NO2��g�� ![]() N2��g��+4CO2��g�� ��H<0���������������£�
N2��g��+4CO2��g�� ��H<0���������������£�
0 min | 5 min | 10 min | 15 min | 20 min | |
c��NO2��/ mol��L-1 | 2.0 | 1.7 | 1.56 | 1.5 | 1.5 |
C��N2��/ mol��L-1 | 0 | 0.15 | 0.22 | 0.25 | 0.25 |
��5l0min����N2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ__________________��
�����±�����˵���÷�Ӧ���ﵽƽ��״̬����________��
A.������ɫ���ٱ仯 B.�����ܶȲ��ٱ仯 C.����ƽ����Է����������ٱ仯
��20 minʱ�������¶Ȳ��䣬�����������м���1.0mol NO2��1.0molCO����t1ʱ�̷�Ӧ�ٴδﵽƽ�⣬��NO2��ת���ʱ�ԭƽ�� ______________������������������С��������������
�ܸ��¶��·�Ӧ�Ļ�ѧƽ�ⳣ��K= __________ ��������λ��Ч��������
��3��CO�����������KOH��Һ�ɹ���ȼ�ϵ����ʯīΪ�缫����
��д���õ�صĸ�����Ӧʽ____________________________��
�ڳ����£��ø�ȼ�ϵ�ص�������Ե缫��1L����ʳ��ˮ����������������������4.48L ������Ϊ��״��������ʱ������Һ��pH=__________����������Һ������仯����֪1g2=0.3��lg5=0.7��
