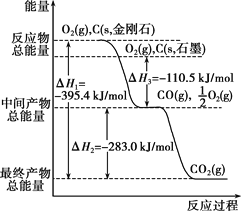
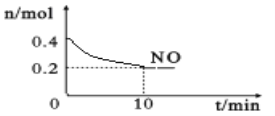
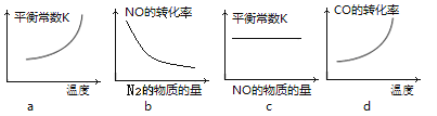
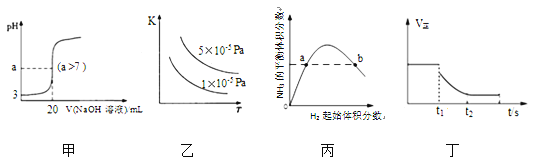
��Ŀ����
����Ŀ��ǰ������Ԫ��X��Y��Z��R��Q�˵���������ӣ�����X��Y��Z��R����Ԫ�صĺ˵����֮��Ϊ58��Y ԭ�ӵ�M��p�����3��δ�ɶԵ��ӣ�Z��Yͬ���ڣ����ڸ������е縺�����Rԭ�ӵ�L�������������������֮��Ϊ4��1����d����еĵ�����������������֮��Ϊ5��2��Q2+�ļ۵����Ų�ʽΪ3d9��
�ش��������⣺
��1��R��̬ԭ�Ӻ�����ӵ��Ų�ʽΪ________��
��2��ZԪ�ص�����������Ӧ��ˮ���ﻯѧʽ��___________��
��3��Y��Z�ֱ���X�γ�����ۻ�����A��B��A��B��ȣ��ȶ��Խϲ����______��д����ʽ����
��4����Q����������Һ��������백ˮ���γ������[Q(NH3)4]SO4��������_________�������ǿռ�ṹ��������[Q(NH3)4]2+�Ľṹ����ʾ��ͼ��ʾΪ_____����λ�������������
���𰸡�1s22s22p63s23p63d54s2 HClO4 PH3 �Ȳ�����ɫ������������ܽ����յõ�����ɫ��Һ 
��������
ǰ������Ԫ��X��Y��Z��R��Q�˵���������ӣ�Y ԭ�ӵ�M��p�����3��δ�ɶԵ���,����Χ�����Ų�Ϊ3s23p3����YΪPԪ�أ�Z��Yͬ����,���ڸ������е縺�������ZΪClԪ�أ�Rԭ�ӵ�L�������������������֮��Ϊ4��1������������Ϊ2,��d����еĵ�����������������֮��Ϊ5��2,d�����ĿΪ5,��Χ�����Ų�Ϊ3d54s2,��RΪMnԪ��,��X��Y��Z��R����Ԫ�صĺ˵����֮��Ϊ58,��X�˵����Ϊ58-15-17-25=1,��XΪHԪ�أ�Q2+�ļ۵����Ų�ʽΪ3d9����Q��ԭ������Ϊ29,��QΪCuԪ�ء�
��1��RΪMnԪ��,��R��̬ԭ�Ӻ�����ӵ��Ų�ʽΪ1s22s22p63s23p63d54s2���𰸣�1s22s22p63s23p63d54s2��
��2��ZΪClԪ�أ�ZԪ�ص�����������Ӧ��ˮ�����Ǹ����ᣬ�仯ѧʽ��HClO4��
��3��YΪPԪ��, ZΪClԪ��, XΪHԪ��,Y��Z�ֱ���X�γ�����ۻ�����ΪPH3��HCl����ǽ�����Cl��P�����ȶ��Դ�СΪHCl> PH3���𰸣�PH3��
��4��QΪCuԪ�أ�����������ҺΪCuSO4��Һ��������백ˮ����Cu(OH)2��ɫ����,�����μӣ��������ܽ��γ������[Cu(NH3)4]SO4������ɫ��Һ�������ǿռ�ṹ��������[ Cu (NH3)4]2+�Ľṹ����ʾ��ͼ��ʾΪ ���𰸣��Ȳ�����ɫ������������ܽ����յõ�����ɫ��Һ��
���𰸣��Ȳ�����ɫ������������ܽ����յõ�����ɫ��Һ�� ��
��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�